The electronic structure of the atom
Before the beginning of the 20th century, it was believed that the atom was the smallest particle that could exist. After 1897, smaller particles were identified (Barrio Gómez de Agüero, 2012). As you saw in the previous section, the atom is composed of particles that can have an electronic charge (positive or negative), or be electrically neutral. These particles may also have mass, or be so light, for our purposes, we can say they have no mass. A neutral atom has equal numbers of positive and negative particles, so overall there is no charge..
Task 4b:
Under a suitable heading, copy the following table into your NSD and complete with the correct charge and mass for each sub-atomic particle. Copy the sentence in bold, above, below the table.
You should be able to remember all of this information for the exam, so test yourself and your classmates when you have completed it.
Task 4b:
Under a suitable heading, copy the following table into your NSD and complete with the correct charge and mass for each sub-atomic particle. Copy the sentence in bold, above, below the table.
You should be able to remember all of this information for the exam, so test yourself and your classmates when you have completed it.
Particle |
Mass |
Charge |
Proton |
. |
. |
Neutron |
. |
. |
Electron |
. |
. |
Explore the structure of an atom with the following animations:
Task 4c: Complete the definitions below in your NSD:
1. Matter is anything that has and takes up .
2. An Atom is the smallest unit of
3. Protons are the sub-atomic particles that are - charged and situated in the nucleus of the atom.
4. Neutrons are the sub-atomic particles that are - charged and situated in the nucleus of the atom.
5. Electrons are .
1. Matter is anything that has and takes up .
2. An Atom is the smallest unit of
3. Protons are the sub-atomic particles that are - charged and situated in the nucleus of the atom.
4. Neutrons are the sub-atomic particles that are - charged and situated in the nucleus of the atom.
5. Electrons are .
Atomic Number (Z)
The atomic number (Z), is the number of protons found in the nucleus of an atom. This determines the identity of the atom, an atom where Z=6 is always carbon. Changing the atomic number would change the element. Elements are ordered according to their atomic number in the periodic table.
In their natural state, atoms are neutral. This means that they have the same number of protons and electrons. Therefore, in a neutral atom the atomic number also indicates the number of electrons.
Z = atomic number = number of protons = number of electrons in neutral atoms.
In their natural state, atoms are neutral. This means that they have the same number of protons and electrons. Therefore, in a neutral atom the atomic number also indicates the number of electrons.
Z = atomic number = number of protons = number of electrons in neutral atoms.
Mass Number (A)
The mass number of an atom is the number of particles the atom contains in its nuclues. . It is therefore, the sum of the number of protons and the number of neutrons.
It is represented with the letter A.
A = number of protons + number of neutrons
If we call the number of neutrons n, we conclude that
A = Z + n
Therefore, if we know Z (the atomic number) and A (the mass number). then we know or can work out the number of protons, neutrons and electrons in that atom.
It is represented with the letter A.
A = number of protons + number of neutrons
If we call the number of neutrons n, we conclude that
A = Z + n
Therefore, if we know Z (the atomic number) and A (the mass number). then we know or can work out the number of protons, neutrons and electrons in that atom.
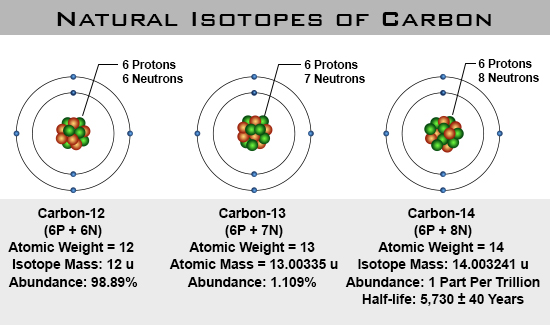
Isotopes
The number of protons and electrons is always the same in all neutral atoms of a chemical element, but the number of neutrons can vary. Atoms with the same number of protons but different numbers of neutrons are called isotopes.
For example: Carbon has 3 different naturally occurring isotopes:
For example: Carbon has 3 different naturally occurring isotopes:
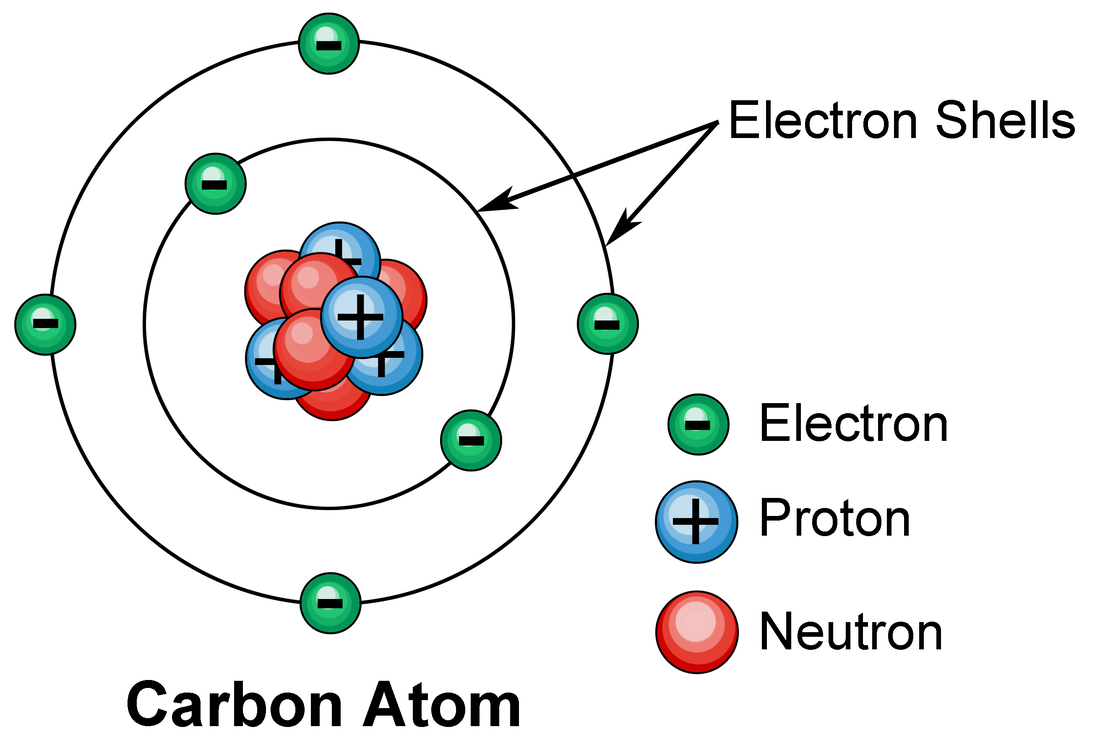
Electron Shells
Electrons in atoms orbit in energy levels, also called electron shells, around the nucleus. The shells can only hold a certain number of electrons. The electrons in an atom occupy the lowest available energy level first. This is the shell nearest the nucleus. When this shell is full the electrons begin to occupy the next shell.
The table below shows the maximum number of electrons an element can have for each of its energy level shells.
The table below shows the maximum number of electrons an element can have for each of its energy level shells.
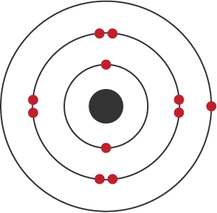
The electronic structure of an atom can be predicted from its atomic number. For example, the atomic number of sodium is 11. Sodium atoms have 11 protons and so 11 electrons:
- two electrons occupy the first shell
- eight electrons occupy the second shell
- one electron occupies the third shell
- each shell is shown as a circle
- each electron is shown as a dot or a cross
The electronic structure of an element is linked to its position on the periodic table.
Number of shells = Period number
Number of electrons in the outer shell = Group number
Total number of electrons (in a neutral atom) = number of protons (atomic number)
The electronic structure of sodium (2,8,1) shows that sodium
Number of shells = Period number
Number of electrons in the outer shell = Group number
Total number of electrons (in a neutral atom) = number of protons (atomic number)
The electronic structure of sodium (2,8,1) shows that sodium
- is in period 3
- is in group 1
- has an atomic number of (2 + 8 + 1) = 11
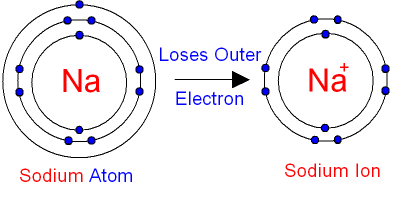
Ions

Atoms have the same number of protons and electrons, and therefore are electrically neutral. However, if an atom loses or gains electrons, the overall charge will change and will be unbalanced..
If the charge is not neutral, the atom has become an ion.
A positive ion is called a cation, and a negative ion is known as an anion.
Atoms form ions as a way to get a full outer shell of electrons. All atoms "want" to have a full outer shell to be like the Nobel gasses.. To do this they will either fill a nearly full shell or empty a shell that has only a few electrons in it. Whichever involves moving the least number of electrons.
For example sodium (Na) has 1 electron in its outer shell. It could try and get 7 more electrons to fill the shell but it is easier to lose 1 electron and have the full 2nd shell become the outer shell.
If the charge is not neutral, the atom has become an ion.
A positive ion is called a cation, and a negative ion is known as an anion.
Atoms form ions as a way to get a full outer shell of electrons. All atoms "want" to have a full outer shell to be like the Nobel gasses.. To do this they will either fill a nearly full shell or empty a shell that has only a few electrons in it. Whichever involves moving the least number of electrons.
For example sodium (Na) has 1 electron in its outer shell. It could try and get 7 more electrons to fill the shell but it is easier to lose 1 electron and have the full 2nd shell become the outer shell.
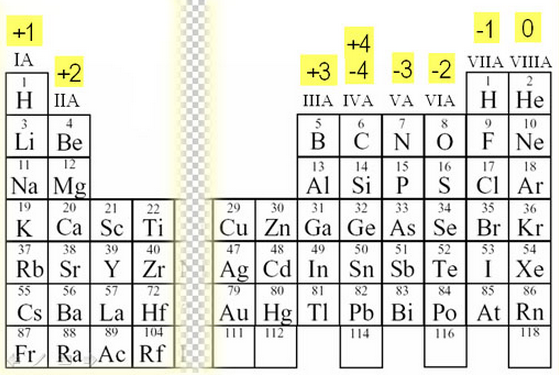
Oxidation numbers
Oxidation numbers do not have anything to do with oxygen. Chemists use oxidation numbers to keep track of the number of electrons an ion has..
A positive number means that the atom has fewer electrons than protons..
A negative number means that the atom has more electrons than protons.
Calcium has a +2 charge, which means it lost two electrons.
Oxygen has a -2 charge, which means it gained two electrons
The oxidation numbers relate to the groups of the periodic table because they depend on the usual number of electrons in the outer shell that an atom has. Elements in the same group will usually have the same oxidation numbers.. Some elements, especially the transition metals can have many different oxidation numbers.
A positive number means that the atom has fewer electrons than protons..
A negative number means that the atom has more electrons than protons.
Calcium has a +2 charge, which means it lost two electrons.
Oxygen has a -2 charge, which means it gained two electrons
The oxidation numbers relate to the groups of the periodic table because they depend on the usual number of electrons in the outer shell that an atom has. Elements in the same group will usually have the same oxidation numbers.. Some elements, especially the transition metals can have many different oxidation numbers.
|
|
|
Task 4d: Complete the definitions below about atoms in your NSD.
1. The Atomic Number is the number of .
2. The Mass Number is the total number of .
3. An Ion is a charged that has either lost or gained negatively- charged
.
4. A Negative Ion is a charged atom that has electrons. It is known as an .
5. 10. A Positive Ion is a charged atom that has electrons. It is known as a .
6. An Element is a pure substance that is made of only type of atom (e.g. gold, oxygen).
1. The Atomic Number is the number of .
2. The Mass Number is the total number of .
3. An Ion is a charged that has either lost or gained negatively- charged
.
4. A Negative Ion is a charged atom that has electrons. It is known as an .
5. 10. A Positive Ion is a charged atom that has electrons. It is known as a .
6. An Element is a pure substance that is made of only type of atom (e.g. gold, oxygen).
References
Barrio Gómez de Agüero, J. (2012). Natural sciences, ESO 2. [San Fernando de Henares]: Oxford Educación.
Moodle.fct.unl.pt,. (2015). Retrieved 6 July 2015, from http://moodle.fct.unl.pt/pluginfile.php/61092/mod_book/chapter/948/Yr_8/chemestry/atoms_and_elements/the_atom.swf
Ntu.ac.uk,. (2015). Retrieved 6 July 2015, from https://www.ntu.ac.uk/cels/outreach/Resources/Secondary/38632.swf
Barrio Gómez de Agüero, J. (2012). Natural sciences, ESO 2. [San Fernando de Henares]: Oxford Educación.
Moodle.fct.unl.pt,. (2015). Retrieved 6 July 2015, from http://moodle.fct.unl.pt/pluginfile.php/61092/mod_book/chapter/948/Yr_8/chemestry/atoms_and_elements/the_atom.swf
Ntu.ac.uk,. (2015). Retrieved 6 July 2015, from https://www.ntu.ac.uk/cels/outreach/Resources/Secondary/38632.swf