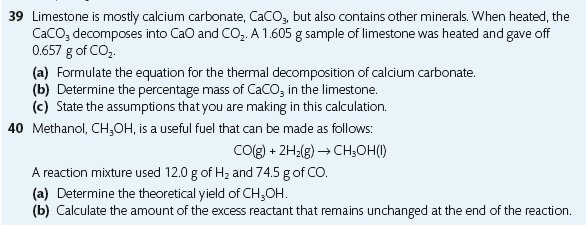Unit 5 - Chemical reactions and chemical equations: StoichiometryKey concept - Change- How important is the rearrangement of matter in chemical reactions?
Related concepts - Consequences - How does science utilise the consequences of chemical reactions? Are the consequences always positive? Global concept - Globalisation and sustainability - In what ways does improving knowledge of chemical reactions impact our goals of global sustainability? |
|
Keywords:
|
Pre-knowledge
The mole
When describing things of a very small size, we often have to use VERY large numbers. For example:
60200000000000000000000000000
Clearly dealing with numbers this big is not very easy. For this reason we sometimes use scientific notation:
6.02 x 10^23
It is even easier, however, to use the unit created by Amadeo Avogadro called the "mole".
1
60200000000000000000000000000
Clearly dealing with numbers this big is not very easy. For this reason we sometimes use scientific notation:
6.02 x 10^23
It is even easier, however, to use the unit created by Amadeo Avogadro called the "mole".
1
The mole is simply a unit that represents 6 .02 x 10^23 of something (atoms, molecules, eggs,hehe). We tend to use it to describe the number of particles but it could be used for anything..
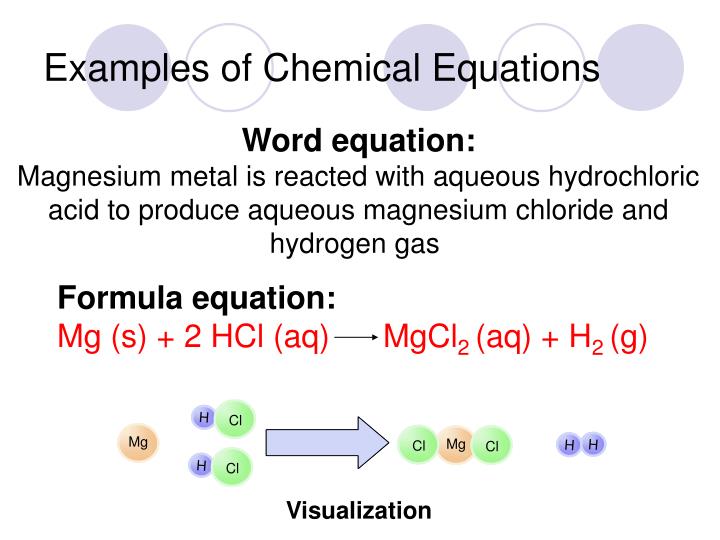
5a 1. Chemical Reactions and Equations
|
A chemical reaction describes chemical changes.
Signs of a chemical change: 1. In a chemical change one or more new chemical substance are produced.. 2. Energy is taken in (endothermic reaction) or given out (exothermic reaction) during the reaction. 3. Te change is usually difficult to reverse. Chemical reactions are represented by chemical equations. C + O2 ---> CO2 Reactants ----> Product 5a 2. Lavoisier´s Law - "The Law of the Conservation of mass"
|
Lavoisier proved that in a chemical reaction, mass cannot be created or destroyed. The three photos below show an example of this. A chemical reaction takes place in the flask on the right of the balance and a mass remains constant on the left. You can see that the balance does not move before, during or after the reaction.
This means that in a chemical reaction we must always account for all of the atoms before and after by balancing it.
Problem 1:
In the following reaction, A + B --> C + D, you completely react 3 .10 g of compound A with 4 .90 g of compound B..
If 2 .35 g of compound C are formed. How much of compound D should be formed?
In the following reaction, A + B --> C + D, you completely react 3 .10 g of compound A with 4 .90 g of compound B..
If 2 .35 g of compound C are formed. How much of compound D should be formed?
Write down your answer:
Problem 2:
Dr Cano measures 5 .00 g of hydrochloric acid into a beaker that has a mass of 24 .00 g. She then adds 2 .50 grams of magnesium.. After the reaction has finished, she records the mass of the beaker with the products in it and finds that the total mass is 30 .70 g. What is the problem with this result? Can you explain it?
Dr Cano measures 5 .00 g of hydrochloric acid into a beaker that has a mass of 24 .00 g. She then adds 2 .50 grams of magnesium.. After the reaction has finished, she records the mass of the beaker with the products in it and finds that the total mass is 30 .70 g. What is the problem with this result? Can you explain it?
Answer: To write your answer, first write down the chemical equation. Consider the reaction carefully e down your final answer.
Balancing equations - This is essential to this topic! Try the questions below:
If you find these difficult then try using the tool below:
|
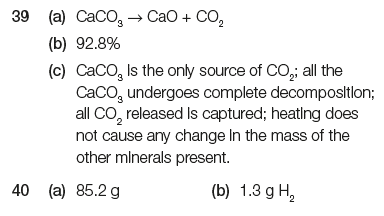
Task 5i:
a. What do these state symbols mean - (s), (l), (g), (aq)? b. In your PCD, balance the equations involved in the three steps of making sulfuric acid (Contact Process) and add the state symbols after each compound: S + O2 → SO2 SO2 + O2 ⇌ SO3 H2O + SO3 → H2SO4 Helpful LINK |
Extension: The second reaction contains a "⇌" symbol instead of an "-->". This symbol is actually used in many chemical reactions.
a. What does this symbol mean the second reaction? Use this source to answer this and the following questions - LINK.
b. In which ways could we change the conditions in this reaction to produce more SO3.
a. What does this symbol mean the second reaction? Use this source to answer this and the following questions - LINK.
b. In which ways could we change the conditions in this reaction to produce more SO3.
5a 3. Types of chemical reactions
There are different types of chemical reactions, You will need to be familiar with the ones below:
.
Synthesis or Combination Reaction: two or more substances react to form just one compound.
Ex: Fe(s) + S(s) ---> FeS(s)
Decomposition Reaction: a substance breaks down into simpler substances.
Ex: 2 AgCl(s) ----> 2 Ag(s) + 2 Cl(g)
Displacement Reaction: Here an element swops places with another element, in a compound.
Ex: 2 HCl(aq) + Zn(s) ----> ZnCl2(aq) + H2(g)
Ex: Na2CO3(aq) + CaCl2(aq) → CaCO3(s) + 2 NaCl(aq)
Combustion Reactions: for our purposes and in this class we will define them as those reactions of hydrocarbon fuels with oxygen, producing carbon dioxide and water as products.
Ex: C5H12(l) + 8 O2(g) → 5 CO2(g) + 6 H2O(l)
Neutralisation Reactions: where acids react with bases giving a salt and water.
Ex: HCl(ag) + NaOH(aq) ---> NaCl(aq) + H2O(i)
.
Synthesis or Combination Reaction: two or more substances react to form just one compound.
Ex: Fe(s) + S(s) ---> FeS(s)
Decomposition Reaction: a substance breaks down into simpler substances.
Ex: 2 AgCl(s) ----> 2 Ag(s) + 2 Cl(g)
Displacement Reaction: Here an element swops places with another element, in a compound.
Ex: 2 HCl(aq) + Zn(s) ----> ZnCl2(aq) + H2(g)
Ex: Na2CO3(aq) + CaCl2(aq) → CaCO3(s) + 2 NaCl(aq)
Combustion Reactions: for our purposes and in this class we will define them as those reactions of hydrocarbon fuels with oxygen, producing carbon dioxide and water as products.
Ex: C5H12(l) + 8 O2(g) → 5 CO2(g) + 6 H2O(l)
Neutralisation Reactions: where acids react with bases giving a salt and water.
Ex: HCl(ag) + NaOH(aq) ---> NaCl(aq) + H2O(i)
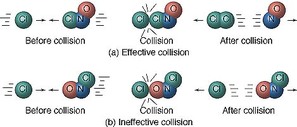
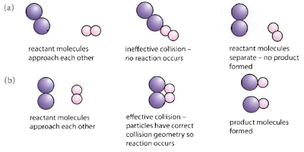
5a.4 Collision theory
For a chemical reaction to happen, the different molecules must:
- Collide with each other - Otherwise they cannot react.
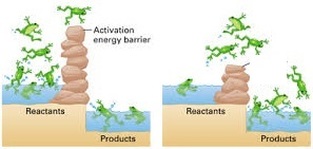
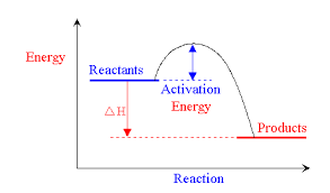
- Enough kinetic energy to react - The minimum amount of energy required is called the "activation energy". If molecules do not have this amount of energy then they cannot react. The diagram below shows a reaction with a high activation energy (left) and a low activation energy (right). You can see that more products are formed on the right.
- The correct orientation - If the two molecules do not collide with each other in the correct way, then they are unlikely to react.
Task 5j:
1. Collision theory describes what happens in chemical reactions. Describe the three aspects of collision theory.
2. Why are reactions with a high activation energy less likely to occur?
3. Most chemical reactions occur between 2 molecules. Why do reactions involving more molecules rare?
1. Collision theory describes what happens in chemical reactions. Describe the three aspects of collision theory.
2. Why are reactions with a high activation energy less likely to occur?
3. Most chemical reactions occur between 2 molecules. Why do reactions involving more molecules rare?
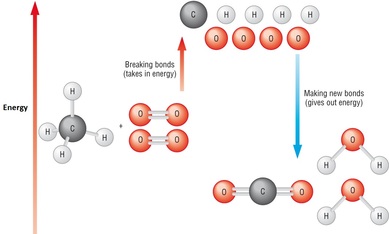
ENERGY AND CHEMICAL REACTIONS
Energy is taken in or given out duing a chemical reaction.
Energy is taken in or given out duing a chemical reaction.
|
|
We can represent chemical reactions as "energy profile diagrams". They show the energy changes when bonds are broken and made. The energy profile diagram below shows an EXOTHERMIC reaction because more energy is released than absorbed.
Task 5k: In the PCD, add the following:
- Your own definition of an exothermic and an endothermic reaction.
- An energy profile diagram for each.
- 2 examples of chemical reactions of each type.
Extension: Read the following pages and animations on EXO/ENDOTHERMIC reactions and reversible reactions. Summarise what you read in 5 sentences. LINK
5a.5 Rates of reaction
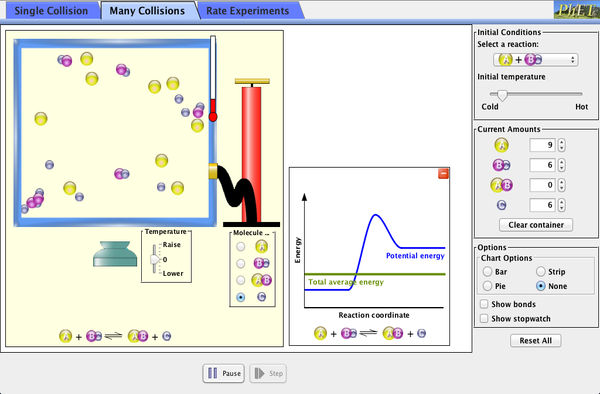
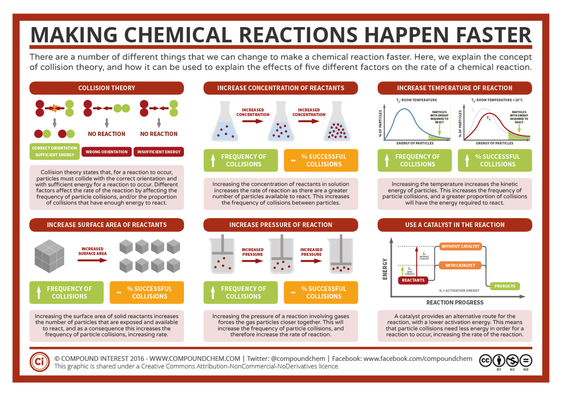
Any way we can increase the number of molecules that pass the three criteria in the section above, the faster the reaction will be. (LINK)
What happens to the rate of reaction when:
- You increase the temperature? - Use LINK
- Increase the pressure/concentration? (So increasing the number of particles in the box) - Use LINK
- Increase the surface area of a reactant? - Watch VIDEO (flour is normally not very reactiove unless it is in powder form)
- You use a catalyst? (This decreases the activation energy of the reaction) - Watch VIDEO
Explanations
Higher temperature --> More kinetic energy --> More collisions and more particles can overcome the activation energy --> More successful reactions --> Higher rate of reaction
Higher pressure/concentration --> More particles --> More collisions --> More reactions --> Higher rate of reaction
Higher surface area of a reactant --> More chance of collisions --> More reactions --> Higher rate of reaction
Use of a catalyst --> Lower activation energy --> More successful reactions --> Higher rate of reaction
Task 5k:
a. Describe three ways that we can increase the rate of a chemical reaction using your knowledge of collision theory.
b. Complete the multiple choice questions below. The ones in red are difficult!
a. Describe three ways that we can increase the rate of a chemical reaction using your knowledge of collision theory.
b. Complete the multiple choice questions below. The ones in red are difficult!
|
| ||||
Reversible reactions and equilibria:
(some are not reversible........)
Dynamic equilibrium
(some are not reversible........)
Dynamic equilibrium
5a.6 Limiting and excess reactants
How many hamburgers can you make using the following ingredients? In each burger must be 1 piece of cheese, 1 piece of lettuce, 1 burger and 2 pieces of tomato.
Hopefully you are able to see that actually we can only make 2 hamburgers as the tomatoes will have run out after making 2.
In this context we would describe the tomatoes as the limiting factor as once they have run out, we cannot make any more burgers. The other ingredients would be in excess as there is some left over (2 buns, 4 pieces of cheese, 2 pieces of lettuce and 3 burgers)..
Stoichiometry with simple chemical reactions
IWe can apply this same principle to chemical reactions. This is the balanced equation for the reaction between hydrochloric acid and sodium hydroxide:
1 : 1 : 1 1
HCl + NaOH --> NaCl + H2O
The reactants and products are in a 1 : 1 : 1 : 1 stoichiometric ratio. This means that if react 1 mole of HCl with 1 mole of NaOH i will produce 1 mole of NaCl and 1 mole of water. However, if i have uneven amount of the reactants then one reactant will run out before the other so the reaction would stop.
1 : 1 : 1 1
HCl + NaOH --> NaCl + H2O
The reactants and products are in a 1 : 1 : 1 : 1 stoichiometric ratio. This means that if react 1 mole of HCl with 1 mole of NaOH i will produce 1 mole of NaCl and 1 mole of water. However, if i have uneven amount of the reactants then one reactant will run out before the other so the reaction would stop.
Using the balanced equation --> NaOH + HCl --> NaCl + H2O
1. How many moles of NaCl would I get if I start with:
a. 3 moles of HCl and 3 moles NaOH? 3 mol NaCl
(As this question provides the perfect amount of reactants to react completely we simply need to compare the stoichiometric ratios of the reactants to the products)
b. 5 moles of HCl and 1 mole NaOH? 1 mol NaCl
(In this question, the 1 mole of NaOH will react with 1 mole of HCl and then the reaction will stop as there is no more NaOH. We describe the NaOH as the limiting reactant as it has run out first and therefore stopped the reaction. As only 1 mol of each reactant has reacted, only 1 mole of NaCl will be produced and there will be 4 mol HCl in excess (remaining unreacted).
c. 5 moles of HCl and 10 moles NaOH? 5 mol NaCl
(In this questions the HCl will run out first after the 5 moles has reacted with 5 moles NaOH so it is the limiting reactant. As only 5 moles of each reactant has reacted, only 5 moles of NaCl will be produced (and 5 mol NaOH in excess).
a. 3 moles of HCl and 3 moles NaOH? 3 mol NaCl
(As this question provides the perfect amount of reactants to react completely we simply need to compare the stoichiometric ratios of the reactants to the products)
b. 5 moles of HCl and 1 mole NaOH? 1 mol NaCl
(In this question, the 1 mole of NaOH will react with 1 mole of HCl and then the reaction will stop as there is no more NaOH. We describe the NaOH as the limiting reactant as it has run out first and therefore stopped the reaction. As only 1 mol of each reactant has reacted, only 1 mole of NaCl will be produced and there will be 4 mol HCl in excess (remaining unreacted).
c. 5 moles of HCl and 10 moles NaOH? 5 mol NaCl
(In this questions the HCl will run out first after the 5 moles has reacted with 5 moles NaOH so it is the limiting reactant. As only 5 moles of each reactant has reacted, only 5 moles of NaCl will be produced (and 5 mol NaOH in excess).
Task 5l: Complete the test below.
Extension:
a. What is a hydrocarbon? What is a combustion reaction?
b. What are the only 2 products in complete combustion of a hydrocarbon fuel?
c. Write a balanced equation for the complete combustion of octane.
d. Add together the stoichiometric coefficients (including any "1´s") and scan the correct answer below to find out if you are correct.
a. What is a hydrocarbon? What is a combustion reaction?
b. What are the only 2 products in complete combustion of a hydrocarbon fuel?
c. Write a balanced equation for the complete combustion of octane.
d. Add together the stoichiometric coefficients (including any "1´s") and scan the correct answer below to find out if you are correct.
5a.7 Calculating the limiting reactant
In reactions where the ratio is not 1 : 1 between the reactants. If i start with 10 moles of both reactant in the following reaction, we have a more complicated situation.
H2SO4 + 2NaOH --> Na2SO4 + 2H2O
To work out the limiting reactant you must compare the amount of each reactant BUT taking into account the stoichiometric ratio. To do this we must divide the number of moles of each reactant by their stoichiometric coefficient (SC) and compare the numbers:
Sulfuric acid = 10 moles ÷ 1 = 10
Sodium hydroxide = 10 moles ÷ 2 = 5
Sodium hydroxide has the proportionally lowest number of moles when we take into account the SC and is therefore the limiting reactant. The sulfuric acid must then be the excess reactant.
H2SO4 + 2NaOH --> Na2SO4 + 2H2O
To work out the limiting reactant you must compare the amount of each reactant BUT taking into account the stoichiometric ratio. To do this we must divide the number of moles of each reactant by their stoichiometric coefficient (SC) and compare the numbers:
Sulfuric acid = 10 moles ÷ 1 = 10
Sodium hydroxide = 10 moles ÷ 2 = 5
Sodium hydroxide has the proportionally lowest number of moles when we take into account the SC and is therefore the limiting reactant. The sulfuric acid must then be the excess reactant.
|
Once we have identified the limiting reactant we use only the number of moles of this reactant to calculate the moles of products.:
H2SO4 + 2NaOH --> Na2SO4 + 2H2O The NaOH is the limiting reactant and we start with 10 moles of it. We can now use the rule of 3 to calculate the amount of products formed: 2 (NaOH) : 1 (Na2SO4) 10 mol : x moles x = 5 mol Na2SO4 2 (NaOH) : 2 (H2O) 10 mol : x moles x = 10 mol H2O |
Task 5m: Balance each equation below, answer the questions and compare with the correct answers.
__ SnO2 + __ H2 --> __ Sn + __ H2O
1. If you use 2 moles of each reactant then what mass of each product will I get?
(Data: Sn - 119, H - 1, O - 16)
__ KOH + __ H3PO4 → __ K3PO4 + __ H2O
2. If you react 10 g of KOH and 20 g H3PO4 what mass of each product will I get?
(Data: K - 40, O - 16, H - 1, P - 31)
3. 216 g of aluminium are made to react with excess hydrochloric acid (forming aluminium chloride and hydrogen). Calculate:
a. The number of grams of salt obtained.
b. The number of moles of acid used up the reaction.
c. If this amount of acid was added to make a solution with a volume of 480 L, what would be the resulting molarity?
d. What volume of hydrogen gas would be produced at 27 oC and 1 atm?
(Data: Al - 27. H - 1, Cl - 35.5 ; Acid + Metal --> Salt + Hydrogen ; R = 0.082)
__ SnO2 + __ H2 --> __ Sn + __ H2O
1. If you use 2 moles of each reactant then what mass of each product will I get?
(Data: Sn - 119, H - 1, O - 16)
__ KOH + __ H3PO4 → __ K3PO4 + __ H2O
2. If you react 10 g of KOH and 20 g H3PO4 what mass of each product will I get?
(Data: K - 40, O - 16, H - 1, P - 31)
3. 216 g of aluminium are made to react with excess hydrochloric acid (forming aluminium chloride and hydrogen). Calculate:
a. The number of grams of salt obtained.
b. The number of moles of acid used up the reaction.
c. If this amount of acid was added to make a solution with a volume of 480 L, what would be the resulting molarity?
d. What volume of hydrogen gas would be produced at 27 oC and 1 atm?
(Data: Al - 27. H - 1, Cl - 35.5 ; Acid + Metal --> Salt + Hydrogen ; R = 0.082)
|
Mixed stoichiometry problems 1
|
5a.8 Percentage yield
|
"Yield" is the amount of a product we obtain
In industry, chemical reactions do not always produce 100% of the amount of products that we calculate. We can describe the amount of product formed by comparing it with how much “theoretically” should have been formed. For example, if a chemical reaction should theoretically give 10 moles of a product but we actually only get 8 moles: Percentage yield = (Experimental yield ÷ theoretical yield) x 100 = (8 ÷ 10) x 100 = 80% yield |
Task 5n:
1. An experiment should theoretically give 0.05 moles of a product. When it was carried out only 0.03 moles were produced. What was the percentage yield?
2. __ Na3PO4 + __ HCl → __ NaCl + __ H3PO4
a. Balance the equation.
b. If i react 0.2 moles of sodium phosphate with 0.3 moles of HCl, what is the limiting reactant?
c. How many moles of NaCl should i produce?
d. When i carry out the experiment i collect 0.15 moles of NaCl. What is the percentage yield?
e. I complete the experiment again and collect 14.6 g NaCl. What is the percentage yield?
(Data: Na - 23 g/mol, Cl - 35.5 g/mol)
3. Mercury(II) oxide thermally decomposes into mercury and oxygen. Given a reaction yield of 80 %, how much mercury oxide was used if 2 L of oxygen were released at 273 °C and 646 Torr?
(Data: 760 Torr = 1 atm, R = 0.082, Hg - 200.5 g/mol, O - 16 g/mol)
1. An experiment should theoretically give 0.05 moles of a product. When it was carried out only 0.03 moles were produced. What was the percentage yield?
2. __ Na3PO4 + __ HCl → __ NaCl + __ H3PO4
a. Balance the equation.
b. If i react 0.2 moles of sodium phosphate with 0.3 moles of HCl, what is the limiting reactant?
c. How many moles of NaCl should i produce?
d. When i carry out the experiment i collect 0.15 moles of NaCl. What is the percentage yield?
e. I complete the experiment again and collect 14.6 g NaCl. What is the percentage yield?
(Data: Na - 23 g/mol, Cl - 35.5 g/mol)
3. Mercury(II) oxide thermally decomposes into mercury and oxygen. Given a reaction yield of 80 %, how much mercury oxide was used if 2 L of oxygen were released at 273 °C and 646 Torr?
(Data: 760 Torr = 1 atm, R = 0.082, Hg - 200.5 g/mol, O - 16 g/mol)
5a. 9 Acids and Bases (pH, and neutralization reactions)
Revision
EXAM HELP: Follow the following steps when dealing with difficult stoichiometry problems:
1. Balance the equation
2. Use the information provided to calculate the moles of both reactants.
3. Calculate the "limiting reactant" by dividing the moles by the coefficient.
4. Use a "rule of 3" to calculate the moles of products.
5. Convert moles of products to mass.
Example:
1. Balance the equation
2. Use the information provided to calculate the moles of both reactants.
3. Calculate the "limiting reactant" by dividing the moles by the coefficient.
4. Use a "rule of 3" to calculate the moles of products.
5. Convert moles of products to mass.
Example:
1. Stoichiometry QR revision task (This task requires you to download a QR code reader - http://_www.codetwo.com/freeware/qr-code-desktop-reader-thanks)
2. Multiple choice stoichiometry questions
|
| ||||
3. Mixed problems 2
4. Theory questions
| theory_questions.docx |
5. Stoichiometry questions
|
| ||||
5. Challenge yourself: (answers at bottom of page)
Challenge yourself answers: