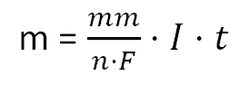Unit 5 - Redox reactions
|
Key concept - Change- How important is the rearrangement of matter in chemical reactions?
Related concepts - Consequences - How does science utilise the consequences of chemical reactions? Are the consequences always positive? Global concept - Globalisation and sustainability - In what ways does improving knowledge of chemical reactions impact our goals of global sustainability? |
Key words
|
|
Pre-knowledge - What are oxidation numbers?
Oxidation numbers are assigned to show (theoretically or actually) the number of electrons lost or gained by an atom or ion in a molecule or compound.
|
Example - Magnesium oxide, MgO (Ionic compound)
This ionic compound is composed of Mg 2+ ions and O 2- ions. As magnesium has lost 2 electrons we assign a +2 oxidation state. As oxygen has gained 2 electrons we assign a -2 oxidation state. (Note: oxidation states must be given with the + or - before the number) |
|
Example - Ammonia, NH3 (covalent compound)
As ammonia is a covalent molecule, we assign oxidation numbers by using the electronegativity values of each atom to predict where the electrons would go if it was to form an ionic compound. As nitrogen is more electronegative than hydrogen, it would take an electron from a hydrogen. As it needs 3 electrons to complete its outer shell, it would need to take an electron from 3 hydrogens as shown in its formula - NH3. As nitrogen has theoretically gained 3 electrons we assign a -3 oxidation state. As each hydrogen has lost 1 electron we assign a +1 oxidation state. |
You have already seen these oxidation states in chemical formulation. This is just the theory behind them.
Task 5a:
- Organise the following into ionic compounds (metal and non-metal ions) and covalent compounds (only non-metals): NaCl, H2O, O2, Mg(NO3)2, NH3, CH4, AgF
- Which of the following numbers could be oxidation states: +1 -2 III 4+ +7 7+ -II 0
- Predict which oxidation states might be likely for the four new elements with atomic numbers 113, 115, 117 and 119.
5.1 Assigning oxidation states
The following rules can be used to help assign ox. states:
- Oxygen = -2 (except in peroxide ion with -1)
- Hydrogen = +1 (except in metal hydrides with -1)
- Group 1, 2 and 3 ions have an ox. state = Group number (e.g. Mg2+ --> +2)
- Group 17 (halides) = -1 (e.g. F- --> -1)
- Anything in its "elemental state" must = 0 (e.g. Ne, O2, N2, Mg, Cl2)
Note: Something in its elemental state is the natural state of a pure element. For example, oxygen is found naturally as the molecule O2 and gold is found naturally as atomic gold, Au .
The sum of the ox. states in a compound/molecule will ALWAYS add up to ZERO.
The sum of ox. states in an ion will ALWAYS add up to the CHARGE on that ion.
The sum of ox. states in an ion will ALWAYS add up to the CHARGE on that ion.
Worked example: Assign oxidation numbers to the following equation: Cl2 + SO2 + 2H2O --> H2SO4 + 2HCl
Cl2 --> In its elemental state so each Cl must be 0 (0 + 0 = 0 )
SO2 --> Oxygen is always -2 therefore sulfur must be +4 (+4 -2 -2 = 0 )
H2O --> Oxygen is always -2 and hydrogen is always +1 (+1 +1 -2 = 0 )
H2SO4 --> Oxygen is always -2 and hydrogen is always +1 so sulfur must be +6 (+1 +1 +6 -2 -2 -2 -2 = 0 )
HCl --> Hydrogen is always +1 and a chloride ion is always -1. (+1 -1 = 0 )
Cl2 --> In its elemental state so each Cl must be 0 (0 + 0 = 0 )
SO2 --> Oxygen is always -2 therefore sulfur must be +4 (+4 -2 -2 = 0 )
H2O --> Oxygen is always -2 and hydrogen is always +1 (+1 +1 -2 = 0 )
H2SO4 --> Oxygen is always -2 and hydrogen is always +1 so sulfur must be +6 (+1 +1 +6 -2 -2 -2 -2 = 0 )
HCl --> Hydrogen is always +1 and a chloride ion is always -1. (+1 -1 = 0 )
0 +4 -2 +1 -2 +1 +6 -2 +1 -1
Cl2 + SO2 + 2H2O --> H2SO4 + 2HCl
Cl2 + SO2 + 2H2O --> H2SO4 + 2HCl
Note: Ox. states can also be represented as roman numerals to prevent confusion with any charges.
0 IV -II I -II I VI -II I -I
Cl2 + SO2 + 2H2O --> H2SO4 + 2HCl
Cl2 + SO2 + 2H2O --> H2SO4 + 2HCl
Task 5b: Quiz
|
| ||||
5.2 Oxidation and reduction
0 +4 -2 +1 -2 +1 +6 -2 +1 -1
Cl2 + SO2 + 2H2O --> H2SO4 + 2HCl
Cl2 + SO2 + 2H2O --> H2SO4 + 2HCl
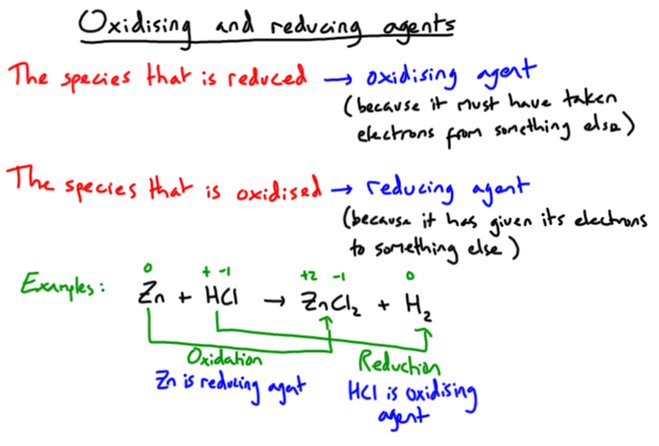
A decrease in ox. state means electrons have been gained --> we call this REDUCTION
An increase in ox. state means electrons have been lost --> we call this OXIDATION
We can remember these processes using the acronym oil rig (above right): "oxidation is loss, reduction is gain"
An increase in ox. state means electrons have been lost --> we call this OXIDATION
We can remember these processes using the acronym oil rig (above right): "oxidation is loss, reduction is gain"
We can also use the terms "oxidising agent" and "reducing agent" to define these species:
Task 5c:
| redox_questions.doc |
Task 5d:
Redox summary video:
5.3 Balancing redox equations
Balancing redox equations consists of being given an unbalanced equation in which redox reactions are occuring. You will have to use the following ion-electron method to carry this out.
5.3a Acidic conditions (using H+)
Example question - Balance the following equation using the ion-electron method (in acidic conditions).
HNO3 + Cu --> Cu(NO3)2 + NO2 + H2O
1. If the equation is written in the molecular form we first have to change it to ionic. This means that:
+1 +5 -2 0 +2 +5 -2 +4 -2 +1 -2
H+ + NO3- + Cu --> Cu2+ + NO3- + NO2 + H2O
2. Assign oxidation states to all elements in the ionic equation and identify which species has been oxidised and which has been reduced (note: we include the whole species that includes the oxidation state changes). Write a half equation for both processes.
NO3- --> NO2 REDUCTION
Cu --> Cu2+ OXIDATION
3. Atoms in each half reaction are balanced, according to this sequence:
a. Atoms other than O and H
b. O atoms, adding the corresponding number of H2O molecules
c. H atoms, added as H+
4. Balance the charges on opposite sides of each half reaction equation by adding electrons to the appropriate side.
NO3- + 2 H+ + e- --> NO2 + H2O
Cu --> Cu2+ + 2 e-
5. The number of electron lost in oxidation must equal the number of electrons gained in the reduction half-reaction. If necessary, multiply each half reaction by the adequate entire number (least common multiple, lcm)
NO3- + 2 H+ + e- --> NO2 + H2O (x2)
Cu --> Cu2+ + 2 e-
-------------------------------------------
2 NO3- + 4 H+ + Cu --> 2 NO2 + 2 H2O + Cu2+
6. Half-reactions are added and common species that appear at both sides of the overall equation are cancelled.
7. Check that mass and charge is balanced.
8. Balanced Ionic Reaction
(Note: You will only need to know how to do this process in acidic conditions. If in basic conditions, we also add OH- ions to eliminate the H+ ions, since H+ + OH- = H2O)
Add the necessary species to rebuild the initial reagents, and simplify if possible.
2 NO3- + 4 H+ + Cu --> 2 NO2 + 2 H2O + Cu2+
2 NO3- 2 NO3-
-------------------------------------------
4 HNO3 + Cu --> 2 NO2 + 2 H2O + Cu(NO3)2
HNO3 + Cu --> Cu(NO3)2 + NO2 + H2O
1. If the equation is written in the molecular form we first have to change it to ionic. This means that:
- For any ionic compounds - we write them down as seperate ions e.g. MgSO4 --> Mg2+ + SO42-
- For and acids - we also break them into sperate ions e.g. HCl --> H+ + Cl-
- For any covalent molecules we leave them as they are e.g. H2O and NO2
+1 +5 -2 0 +2 +5 -2 +4 -2 +1 -2
H+ + NO3- + Cu --> Cu2+ + NO3- + NO2 + H2O
2. Assign oxidation states to all elements in the ionic equation and identify which species has been oxidised and which has been reduced (note: we include the whole species that includes the oxidation state changes). Write a half equation for both processes.
NO3- --> NO2 REDUCTION
Cu --> Cu2+ OXIDATION
3. Atoms in each half reaction are balanced, according to this sequence:
a. Atoms other than O and H
b. O atoms, adding the corresponding number of H2O molecules
c. H atoms, added as H+
4. Balance the charges on opposite sides of each half reaction equation by adding electrons to the appropriate side.
NO3- + 2 H+ + e- --> NO2 + H2O
Cu --> Cu2+ + 2 e-
5. The number of electron lost in oxidation must equal the number of electrons gained in the reduction half-reaction. If necessary, multiply each half reaction by the adequate entire number (least common multiple, lcm)
NO3- + 2 H+ + e- --> NO2 + H2O (x2)
Cu --> Cu2+ + 2 e-
-------------------------------------------
2 NO3- + 4 H+ + Cu --> 2 NO2 + 2 H2O + Cu2+
6. Half-reactions are added and common species that appear at both sides of the overall equation are cancelled.
7. Check that mass and charge is balanced.
8. Balanced Ionic Reaction
(Note: You will only need to know how to do this process in acidic conditions. If in basic conditions, we also add OH- ions to eliminate the H+ ions, since H+ + OH- = H2O)
Add the necessary species to rebuild the initial reagents, and simplify if possible.
2 NO3- + 4 H+ + Cu --> 2 NO2 + 2 H2O + Cu2+
2 NO3- 2 NO3-
-------------------------------------------
4 HNO3 + Cu --> 2 NO2 + 2 H2O + Cu(NO3)2
Click to set custom HTML
|
Example 1:
Balance this equation using the ion-electron method:
Li + CuSO4 --> Li2SO4 + Cu Answer: 2Li + CuSO4 --> Li2SO4 + Cu |
|
|
Example 2:
Balance this equation using the ion-electron method: O2 + H2S --> S + H2O Answer: O2 + 2H2S --> 2H2O + 2S |
|
Balancing redox reaction with Stoichiometry calculation
- Given the chemical reaction: potassium dichromate + hydroiodic acid + perchloric acid, yielding to chromium(III) perchlorate + molecular iodine + potassium perchlorate + water: a) balance the reaction; b) determine which is the oxidant specie; c) calculate the number of grams of iodine obtained in the reaction if 200 cc of 2 M solution of the oxidant are consumed. Cr 52; K 39; I 127.
- Potassium permanganate react with hydrogen sulfide in presence of manganese(II) sulfate, sulfur, potassium sulfate and water: a) balance the corresponding redox equation; b) indicate which is the reductant specie and the anode; c) determine the volume of 0.5 M hydrogen sulfide solution needed if in the process 100 g of sulfur are obtained. S 32, K 39.
- Manganese dioxide and potassium iodide react in presence of sulfuric acid to give manganese(II) sulfate, iodine, potassium sulfate and water: a) balance the given reaction; b) determine the oxidant and the reductant species; c) calculate the maximum number of grams of iodine possible, if we begin with 1 kg de manganese dioxide 95.7 % in richness. Mn 55, K 39; I 127.
- Nitric acid attacks mercury in presence of hydrochloric acid to give nitrogen, mercury(II) chloride and water: a) balance the corresponding redox reaction; b) determine which is the reductant specie and the cathode; c) determine how many litres of water vapour will be given off at 127 °C and 1520 mmHg, it 500 cc of 2 M nitric acid solution are consumed in the process. N 14, Hg 200
5.3b Basic conditions (using OH-)
Balancing redox reactions in basic conditions is almost exactly the same but because we are not in acidic conditions we cannot have H+. Therefore in Step 3 there is one extra thing we must do:
3. Atoms in each half reaction are balanced, according to this sequence:
a. Atoms other than O and H
b. O atoms, adding the corresponding number of H2O molecules
c. H atoms, added as H+
d. Add an equal amount of OH- ions to each side to convert any H+ into H2O. (H+ + OH- --> H2O)
3. Atoms in each half reaction are balanced, according to this sequence:
a. Atoms other than O and H
b. O atoms, adding the corresponding number of H2O molecules
c. H atoms, added as H+
d. Add an equal amount of OH- ions to each side to convert any H+ into H2O. (H+ + OH- --> H2O)
Task 5e: Complete the quiz below
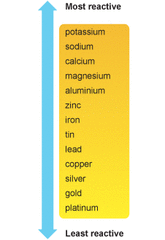
5.4 The reactivity series of metals
|
A REACTIVE METAL LOSES ITS OUTER ELECTRONS MORE EASILY (is oxidised more easily)
Zinc is quite a reactive metal so is oxidised easily when it reacts with an acid: 0 +2 Zn + 2HCl ---> ZnCl2 + H2 Copper is very unreactive so rarely undergoes oxidation: 0 Cu + 2HCl ---> No reaction Different metals have different levels of reactivity as shown in the "Reactivity Series". |
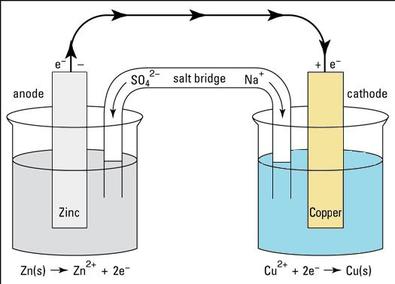
5.5a Electrochemical cells - Galvanic cells
These differences in reactivity actually allow use to produce ELECTRICITY (the flow of electrons) in something called a Galvanic cell. It uses a chemical reaction to produce electricity.
In the diagram below we see an example using a piece of zinc and a piece of copper.
In the diagram below we see an example using a piece of zinc and a piece of copper.
|
Process:
Key features:
|
Mr Canning video explanation - LINK
Simulation: LINK
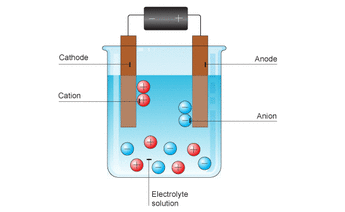
5.5b Electrochemical cells - Electrolysis
The opposite of a Galvanic cell is called and electrolytic cell. This uses electricity to produce a chemical change.
|
Process:
|
|
Uses:
|
Mr Canning video explanation - LINK
Task 5f: Copy and paste this document to your PCD and complete the questions once you have carried out the experiments.
REDOX DEMONSTRATIONS
REDOX DEMONSTRATIONS
5.6a Faraday´s 1st Law
Faraday came up with some very useful equations related to electrolysis. The first helps us calculate the mass of an element formed at one of the electrodes by calculating the number of electrons being passed around the circuit.
Example 1: Calculate the mass of aluminium produced when molten aluminium oxide is electrolysed using a current (I) of 20,000 A, for 5 hours, 21 min and 40s. Data: F = 96500 C/mol; Al - 27 g/mol
mm = 27
n = Al3+ requires 3 electrons to form Al
F = 96500
I = 20000
t = 19300
m = 36000 g
n = Al3+ requires 3 electrons to form Al
F = 96500
I = 20000
t = 19300
m = 36000 g
5.6b Faraday´s 2nd Law
Faraday´s s 2nd Law simply describes how metals with ions with high oxidation states require more electricity than those with low oxidation states.
Example: If the same 12 moles of e- are passed through a Cu + and a Ni 3+ solution. Which will deposit the greatest number of moles of metal?
Answer: The 2 reduction half-equations for forming the metals are:
Cu + + e- ---> Cu Ni 3+ + 3e- ---> Ni
So if we balance the stoichiometric coefficients with the 12 e- being produced:
12Cu+ + 12e- ---> 12Cu 4Ni 3+ + 12e- ---> 4Ni
We can see that 12 mol Cu and 4 mol Ni would be produced.
Example: If the same 12 moles of e- are passed through a Cu + and a Ni 3+ solution. Which will deposit the greatest number of moles of metal?
Answer: The 2 reduction half-equations for forming the metals are:
Cu + + e- ---> Cu Ni 3+ + 3e- ---> Ni
So if we balance the stoichiometric coefficients with the 12 e- being produced:
12Cu+ + 12e- ---> 12Cu 4Ni 3+ + 12e- ---> 4Ni
We can see that 12 mol Cu and 4 mol Ni would be produced.
Revision
Redox equation questions
1. Calculate the oxidation state of each of the following chemical species:
a) NO2, b) HIO3, c) O3, d) O2 , e) O22-
f) (NH4)2Cr2O7, g) NO+, h) PCl3, i) C6H6, j) CH2=CH2,
k) NaHCO3, l) Fe3+, m) Ne, n) Sc2O3, o) OsO4,
p) H2O2, q) PdH4, r) P4, s) AsH3, t) P2O54-
u) NH4+, v) SnI4, w) ICl4- x) K2O2.
2. Which species are oxidising agents and which are reducing agents in the following balanced redox reactions?
a) 2Mg + O2 --> 2MgO
b) CuSO4 + Zn --> ZnSO4 + Cu
c) 2Al + 3Cl2 --> 2AlCl3
d) Cl2 + SO2 + 2H2O --> H2SO4 + 2HCl
e) Zn + 2HCl --> ZnCl2 + H2
f) MnO2 + 4HCl --> MnCl2 + Cl2 + 2H2O
g) KMnO4 + H2O2 + H2SO4 --> MnSO4 + O2 + K2SO4 + H2O
3. Balance the following redox reactions in acidic conditions:
1) HCl + KMnO4 --> Cl2 + KCl + MnCl2 + H2O
2) Cu + HNO3 --> NO + Cu(NO3)2 + H2O
3) Zn + HNO3 --> Zn(NO3)2 + (NH4)NO3 + H2O
4) FeCl2 + H2O2 + HCl --> FeCl3 + H2O
5) O2 + H2S --> S + H2O
6) SO2 + HNO3 + H2O --> H2SO4 + NO
7) NaClO + NaI + HCl --> I2 + NaCl
8) HNO3 + H2S --> NO + S + H2O
9) HNO3 + Fe --> Fe(NO3)3 + NO + H2O
4. Balance the following redox reactions in basic conditions:
1) KClO3 + KOH + Cr(OH)3 --> KCl + K2CrO4 + H2O
1. Calculate the oxidation state of each of the following chemical species:
a) NO2, b) HIO3, c) O3, d) O2 , e) O22-
f) (NH4)2Cr2O7, g) NO+, h) PCl3, i) C6H6, j) CH2=CH2,
k) NaHCO3, l) Fe3+, m) Ne, n) Sc2O3, o) OsO4,
p) H2O2, q) PdH4, r) P4, s) AsH3, t) P2O54-
u) NH4+, v) SnI4, w) ICl4- x) K2O2.
2. Which species are oxidising agents and which are reducing agents in the following balanced redox reactions?
a) 2Mg + O2 --> 2MgO
b) CuSO4 + Zn --> ZnSO4 + Cu
c) 2Al + 3Cl2 --> 2AlCl3
d) Cl2 + SO2 + 2H2O --> H2SO4 + 2HCl
e) Zn + 2HCl --> ZnCl2 + H2
f) MnO2 + 4HCl --> MnCl2 + Cl2 + 2H2O
g) KMnO4 + H2O2 + H2SO4 --> MnSO4 + O2 + K2SO4 + H2O
3. Balance the following redox reactions in acidic conditions:
1) HCl + KMnO4 --> Cl2 + KCl + MnCl2 + H2O
2) Cu + HNO3 --> NO + Cu(NO3)2 + H2O
3) Zn + HNO3 --> Zn(NO3)2 + (NH4)NO3 + H2O
4) FeCl2 + H2O2 + HCl --> FeCl3 + H2O
5) O2 + H2S --> S + H2O
6) SO2 + HNO3 + H2O --> H2SO4 + NO
7) NaClO + NaI + HCl --> I2 + NaCl
8) HNO3 + H2S --> NO + S + H2O
9) HNO3 + Fe --> Fe(NO3)3 + NO + H2O
4. Balance the following redox reactions in basic conditions:
1) KClO3 + KOH + Cr(OH)3 --> KCl + K2CrO4 + H2O
|
ANSWERS
|
| ||
Galvanic and electrolytic cell questions
1. Look a the following reactivity series of metals:
Most reactive Mg > Al > Zn > Fe > Pb > Cu > Ag Least reactive
Which is would be most easily oxidised and which least easily?
2. An electrolysis has taken place for 48 minutes and 15 seconds using a 0.5 A current on a cobalt(III) chloride solution. How much (mass) cobalt will be deposited? (F = 96500 C/mol; Co = 59 g/mol)
3. a) How long will it take to deposit all the silver contained in a 100 mL beaker full of 0.1 M silver nitrate using a 0.1 A current? b) If the same time and current was used for a solution of gold(III) chloride. Would more or less gold be deposited? (Ag = 108 g/mol)
4. Without performing any calculation, answer these questions:
a) Which element will deposit a higher number of moles with the same current and time, copper from copper sulfate or aluminium from aluminium oxide?
b) In order to obtain the same number of moles of copper(II) and aluminium at the time, should I increase or reduce the current through the copper solution?
5. What electrical current should pass through an iron(III) nitrate solution if 28 g of iron are to be deposited in 6 hours? What if we had copper(II) instead of iron(III) nitrate?
Most reactive Mg > Al > Zn > Fe > Pb > Cu > Ag Least reactive
Which is would be most easily oxidised and which least easily?
2. An electrolysis has taken place for 48 minutes and 15 seconds using a 0.5 A current on a cobalt(III) chloride solution. How much (mass) cobalt will be deposited? (F = 96500 C/mol; Co = 59 g/mol)
3. a) How long will it take to deposit all the silver contained in a 100 mL beaker full of 0.1 M silver nitrate using a 0.1 A current? b) If the same time and current was used for a solution of gold(III) chloride. Would more or less gold be deposited? (Ag = 108 g/mol)
4. Without performing any calculation, answer these questions:
a) Which element will deposit a higher number of moles with the same current and time, copper from copper sulfate or aluminium from aluminium oxide?
b) In order to obtain the same number of moles of copper(II) and aluminium at the time, should I increase or reduce the current through the copper solution?
5. What electrical current should pass through an iron(III) nitrate solution if 28 g of iron are to be deposited in 6 hours? What if we had copper(II) instead of iron(III) nitrate?
Answers: 1. Mg most and Ag least. 2. 0.295 g (5x10`-3 mol) 3. a) 9650 s b) less (one third less)
4. a) Higher number for Cu than for Al, b) reduce
4. a) Higher number for Cu than for Al, b) reduce