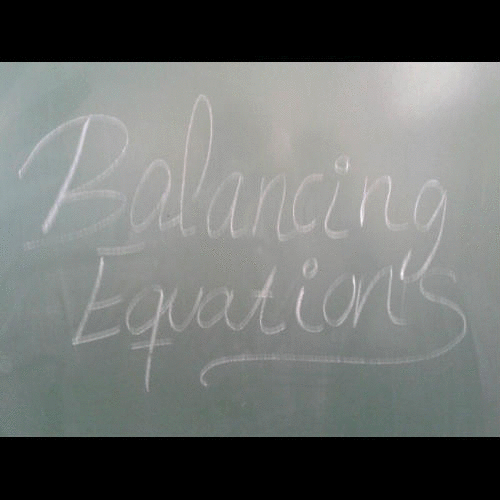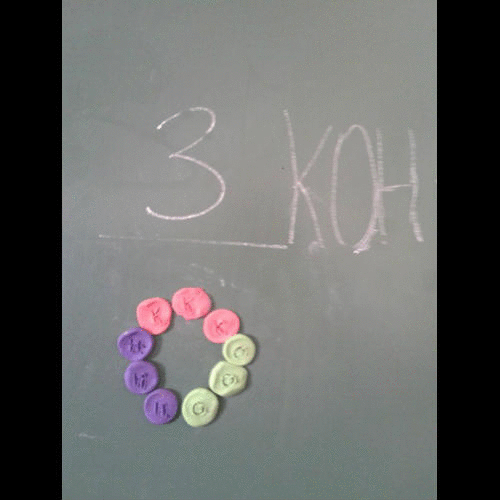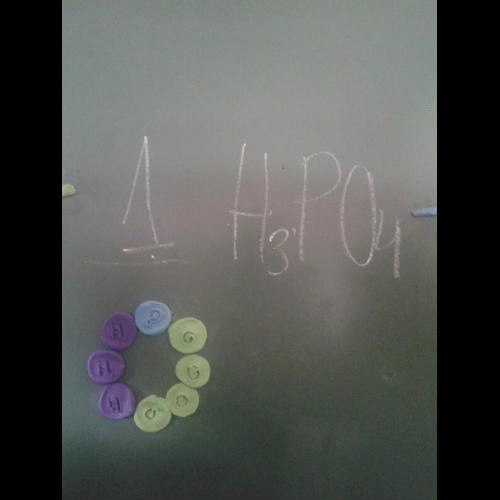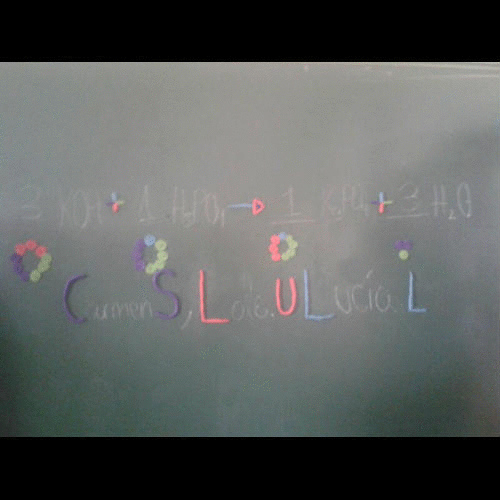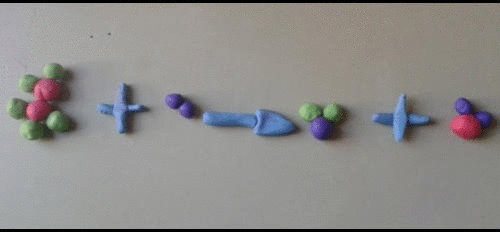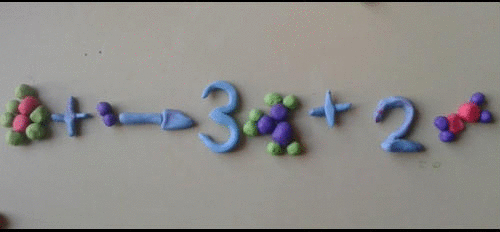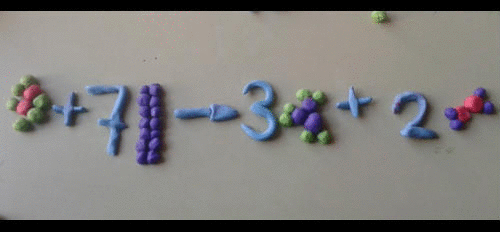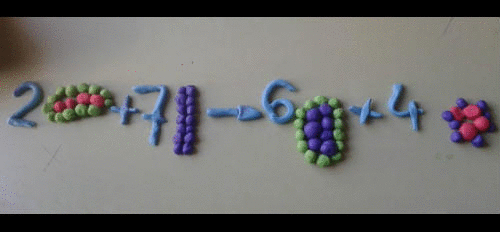Lavoisiers law and balancing equations
The chemicals we have at the start of a reaction are called reactants. When a chemical reaction takes place the atoms in these substances are rearranged to form new chemicals. The substances formed in a reaction are called products.
Key points to remember:
- In chemical reactions new substances are made.
- A reaction can be summarised in a word equation or a symbol equation.
- The total mass of the reactants is the same as the total mass of products in a reaction
- A symbol equation must be balanced as no atoms are created or destroyed.
- You can never change a formula when balancing an equation. You can only put a number in front of a formula.
- No atoms are created or destroyed in a chemical reaction.
- There should be the same number of atoms of each type of element each side of the equation.
Magnesium + Oxygen ----> Magnesium oxide
Student-produced stopmotion animations explaining balancing equations:
Pedro, Jesús, Quico, Iñaki |
Task 4i:
Download here a worksheet with a step by step explanation on how to balance equations
Click here to practice balancing equations
Download here a worksheet with a step by step explanation on how to balance equations
Click here to practice balancing equations