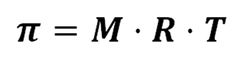Unit 3 - Mixtures and Pure substances
Colligative properties
Key concept - Change- How do the compositions of solution relate to their properties?
Related concepts - Consequences - How does science utilise the consequences of chemical reactions? Are the consequences always positive?
Global concept - Globalisation and sustainability - In what ways does improving knowledge of chemical reactions impact our goals of global sustainability?
Related concepts - Consequences - How does science utilise the consequences of chemical reactions? Are the consequences always positive?
Global concept - Globalisation and sustainability - In what ways does improving knowledge of chemical reactions impact our goals of global sustainability?
|
Keywords
|
|
Pre-knowledge
Task 3a: Complete this online pre-knowledge quiz:
Reminder: Concentrations
Colligative properties are properties of solutions that depend only on the number of number of particles of solute and solvent in the solution, not the nature of the solute. (Bodner, 2014)
For this reason we must have a good understanding of different ways of representing the number of solute and solvent particles present. These representations are called concentrations.
For this reason we must have a good understanding of different ways of representing the number of solute and solvent particles present. These representations are called concentrations.
Concentrations are used to describe how much of a solute is present compared with the amount of solvent or solution.
The amount of a substance frequently requires you to calculate the number of moles (n) of a substance:
Moles = mass (g) / molecular mass (g/mol)
We will see 4 units of concentration used in the topic of colligative properties (and some variations using these units).
1. Molarity (M) = moles of solute (mol) / litres of solution (L) Units = M or mol/L
2. Molality (m) = moles of solute (mol) / mass of solvent (kg) Units = m or mol/kg
3. Molar fraction (xi) = moles of one substance / total moles in mixture Units = no units
4. Mass concentration = mass of solute / litres of solution Units = g/L
You will be required to interconvert these units, for example: Convert a concentration of 40 g/L of NaOH solution into molarity (mol/L):
Problems on concentration: (Click Here)
The amount of a substance frequently requires you to calculate the number of moles (n) of a substance:
Moles = mass (g) / molecular mass (g/mol)
We will see 4 units of concentration used in the topic of colligative properties (and some variations using these units).
1. Molarity (M) = moles of solute (mol) / litres of solution (L) Units = M or mol/L
2. Molality (m) = moles of solute (mol) / mass of solvent (kg) Units = m or mol/kg
3. Molar fraction (xi) = moles of one substance / total moles in mixture Units = no units
4. Mass concentration = mass of solute / litres of solution Units = g/L
You will be required to interconvert these units, for example: Convert a concentration of 40 g/L of NaOH solution into molarity (mol/L):
Problems on concentration: (Click Here)
3a.1 Colligative properties introduction |
|
|
|
3a.2 Boiling point elevation (ΔTb)
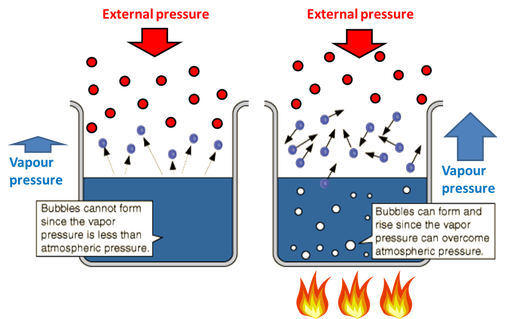
For a liquid to boil, the vapour pressure of the liquid must overcome the external air pressure. When this happen it allows all molecules in the liquid to vaporize and hence, we see bubbles.
|
When a liquid has not reached its boiling point, the vapour pressure is lower than the external pressure. This means the external pressure is able to keep the liquid in the liquid state.
|
When heated to the boiling point, the vapour pressure is now greater than the external pressure so is able to overcome it. Therefore all the molecules in the liquid can now turn into a gas.
|
Raoult´s Law states that adding a non-volatile solute to a solvent will always decrease vapour pressure. If we apply this to boiling points then we know:
Experimentally, we know that the change in boiling point of the solvent above a solution from that of the pure solvent is directly proportional to the molal concentration (molality) of the solute:
- A solution will have a lower vapor pressure than the pure solvent.
- So more heat will be required to overcome external pressure.
- So boiling point will always increase when a solute is added.
Experimentally, we know that the change in boiling point of the solvent above a solution from that of the pure solvent is directly proportional to the molal concentration (molality) of the solute:
|
ΔBP = Kb x m
|
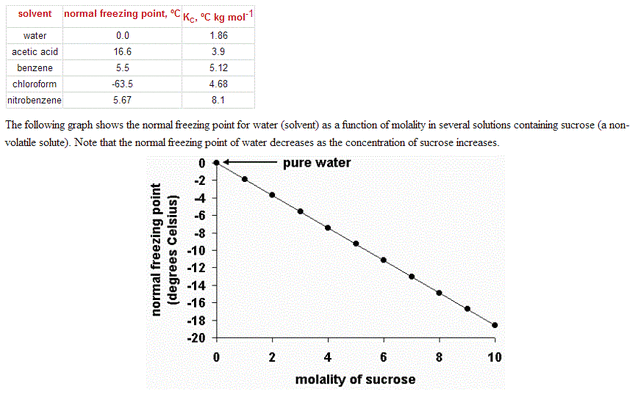
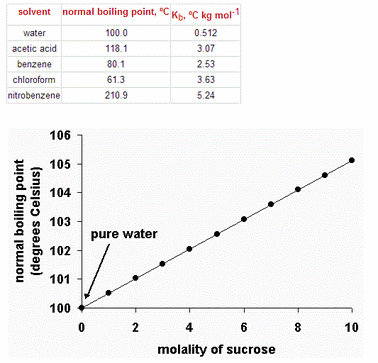 This graph shows the normal boiling point for water (solvent) as a function of molality in solutions containing sucrose (a non-volatile solute). Note that the normal boiling point of water increases as the concentration of sucrose increases.
This graph shows the normal boiling point for water (solvent) as a function of molality in solutions containing sucrose (a non-volatile solute). Note that the normal boiling point of water increases as the concentration of sucrose increases.
ΔBP is the elevation in boiling point (always an increase) of the solution,
Kb is the ebulloscopic constant
m is the molality of the solute in the solution.
Note: the ebulloscopic constant, Kb, has a specific value depending on the identity of the solvent. You do not need to learn these:
Kb for water = 0.512 ºC kg /mol
Task 3g:
- Describe the relationship between the boiling point and molaity of the solution.
- What is the ebullioscopic constant for water?
- What is the boiling point elevation when 11.4 g of ammonia (NH3) is dissolved in 200 g of water? Kb for water is 0.512 °C/m. (Hint: First work out the molality of the solution)
- When dissolving 5 g of a certain solute in 50 g of water, the resulting solution boils at 100.5 °C. What is the corresponding molecular mass of the solute?

Extension: Why do we often add salt to water before cooking things?
Online questions and answers - LINK
3a.3 Freezing point depression (ΔTf)

In the opposite manner to boiling point elevation, the freezing point of a solvent will always decrease when we add a solute. The process of freezing requires particles of a liquid to form a regular structure (as shown in the diagram below). This occurs by removing energy from the particles until they can form this regular structure.
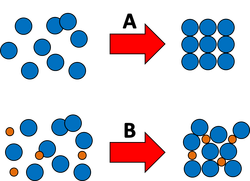
With a pure solvent, this process occurs relatively easily as all of the particles are of the same size and shape as each other.
However, if we add a solute to the liquid, it becomes more difficult for the particles to form a regular structure and therefore we must cool the solution more than normal (remove more energy) to acheive a solid state.
With a pure solvent, this process occurs relatively easily as all of the particles are of the same size and shape as each other.
However, if we add a solute to the liquid, it becomes more difficult for the particles to form a regular structure and therefore we must cool the solution more than normal (remove more energy) to acheive a solid state.
This relationship is represented in the following equation:
ΔFP = Kc x m
ΔFP is the depression in freezing point (always a decrease) of the solution,
Kc is the cryoscopic constant
m is the molality of the solute in the solution.
Note that the cryoscopic constant, Kb, has a specific value depending on the identity of the solvent. You do not need to learn these:
Kc for water = 1.86 ºC kg /mol
ΔFP = Kc x m
ΔFP is the depression in freezing point (always a decrease) of the solution,
Kc is the cryoscopic constant
m is the molality of the solute in the solution.
Note that the cryoscopic constant, Kb, has a specific value depending on the identity of the solvent. You do not need to learn these:
Kc for water = 1.86 ºC kg /mol
Task 3h:
- What is the new freezing point if a solution of water is made with 2 moles of sugar in 500 g water? The freezing point of pure water is 0 ºC and the cryscopic constant is 1.86 ºC/m. (Hint: first work out the molality of the solution)
- Calculate how much ethanol (C2H5OH) could be needed per litre of water in order not to freeze above -4.8 °C.
- When 0.258 g of a molecular compound, benzoic acid, was dissolved in 40.0 g of benzene, the freezing point of the solution was lowered to 5.23 °C. What is the molecular weight of the benzoic acid?
|
Online questions and answers - LINK
|

Extension: If very cold weather is predicted after a period of wet weather people spread salt (NaCl) on the roads. Why might they do this?
|
IMPORTANT POINT: Why can we use ºC or K with BP elevation and FP depression?
With the two equations above we are talking not about temperature alone but change in temperature. As the gaps between the units are the same, a change of 10 ºC (for example) is equal to a change of 10 K. This is shown in the diagram to the right where a difference of 100 units is the same in both degrees (0-100 ºC) and kelvin (273-373 K).
3a.4 Vapour pressure and Raoult´s Law
See Raoult´s Facebook page --> LINK
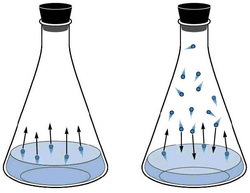
If we take an Erlenmeyer flask with some water and remove all of the air inside, due to the Maxwell-Boltzmann distribution, some molecules of water on the surface will have enough energy to vaporize. The pressure caused by this gas is called vapor pressure.
"The vapour pressure of a liquid is the pressure of a vapour in equilibrium with the liquid phase."(Chem.purdue.edu, 2014)
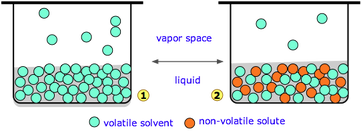
If we dissolve a non-volatile solute in the liquid, we find that the vapour pressure will always decrease. (Non-volatile means that the solute will not itself vaporize and affect the vapor pressure e.g. sugar or salt)
"The vapour pressure of a liquid is the pressure of a vapour in equilibrium with the liquid phase."(Chem.purdue.edu, 2014)
If we dissolve a non-volatile solute in the liquid, we find that the vapour pressure will always decrease. (Non-volatile means that the solute will not itself vaporize and affect the vapor pressure e.g. sugar or salt)
For an ideal solution, with a solvent (A) and one non-volatile solute (B), the equilibrium vapour pressure is given by Raoult's law:
Vapor pressure of solution (P) = Vapor pressure of pure solvent (Po) x Molar fraction of solvent (Xo)
As discussed in the defintion of colligative properties, it does not matter what the solute is, only the number of moles is important.
Task 3f:
1. At 25 ºC, pure water has a vapour pressure of 3.2 kPa. What is the vapour pressure of these 3 sugar solutions at 25 ºC (First work out the molar fraction of the solvent):
a) 1 moles sugar in 30 moles water.
b) 0.02 moles sugar in 0.06 moles water.
c) A 4 molal solution. (Hint: how many moles of sugar in would be in 1 kg water? How many moles of water in 1 kg water)
d) 3.42 g sugar (sucrose) in 180 g water.
Extension:
I dissolve 1 mol of NaCl, MgCl2 and AlCl3 into 3 seperate beakers with 1 L water. Which solution would have the lowest vapor pressure?
1. At 25 ºC, pure water has a vapour pressure of 3.2 kPa. What is the vapour pressure of these 3 sugar solutions at 25 ºC (First work out the molar fraction of the solvent):
a) 1 moles sugar in 30 moles water.
b) 0.02 moles sugar in 0.06 moles water.
c) A 4 molal solution. (Hint: how many moles of sugar in would be in 1 kg water? How many moles of water in 1 kg water)
d) 3.42 g sugar (sucrose) in 180 g water.
Extension:
I dissolve 1 mol of NaCl, MgCl2 and AlCl3 into 3 seperate beakers with 1 L water. Which solution would have the lowest vapor pressure?
Vapour pressure and Raoult´s Law:
| vapour_pressure_questions_and_answers.docx |
3a.5 Osmotic pressure (π)
|
What is osmosis?
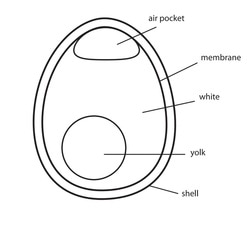
Osmosis is the movement of a solvent from an area of a low concentration (solution) to an area of high concentration (solution) through a semi-permeable membrane (that only allows the movement of the solvent). In effect, this process happens until an equal concentration is acheived on each side of the membrane.
|
|
We can see this process occurring using a simple experiment with eggs. An egg is actually surrounded by 2 layers - the shell, and a semi-permeable membrane. We can use acid to dissolve the shell but it will not dissolve the semi-permeable membrane.
We can do this to 2 eggs and place each one in a different solution:
|
The after 24 hours, the eggs are removed and shown below:
- In the distilled water (hypotonic) example, water has passed into the egg as it has a high concentration of solutes. It gets bigger!
- With the salt water, water has left the egg and moved to the higher concentration of the salt water. It gets smaller!
Osmotic pressure (π) is the pressure required to prevent this process from happening. It can be calculated using this equation:
π = osmotic pressure, M = molarity, R = Gas constant (0.082), T = temperature
As we know that M = mass (g) / molar mass we can substitute this into the equation to calculate mass or molar mass as well.
Again we see that the nature of the solute does not matter, only the number of moles. (Goldbook.iupac.org, 2014)
Again we see that the nature of the solute does not matter, only the number of moles. (Goldbook.iupac.org, 2014)
Don César´s video questions and answers:
3a.6Revision
Mixed colligative properties problems:
| colligative_properties_questions.docx |
EXAM HELP: For problems with colligative properties try to always write 3 related equations:
1. Identify the colligative property equation --> Vapour pressure/Boiling point/Freezing point/Osmotic pressure
2. Identify the concentration equation in it --> Molality (m)/Molarity (M)/Molar fraction (x)
3. Write the equation for moles --> Moles = mass/mm
You will then be able to work through each of the three equations until you get the final result. (You may not need all three).
1. Identify the colligative property equation --> Vapour pressure/Boiling point/Freezing point/Osmotic pressure
2. Identify the concentration equation in it --> Molality (m)/Molarity (M)/Molar fraction (x)
3. Write the equation for moles --> Moles = mass/mm
You will then be able to work through each of the three equations until you get the final result. (You may not need all three).
Vaporization
|
|
What is the difference between evaporation and boiling?
These 2 processes are both types of vaporisation.
|
Evaporation
Occurs at any temperature between freezing point and boiling point because there will always be a small percentage of particles that can overcome to IMFs and vaporise (see below).
|
Boiling
Occurs at temperatures above the boiling point when all molecules have sufficient energy to overcome IMFs and can all vaporise.
|
Water boiling very quickly a water container being thrown into lava --> VIDEO
Extension: Why might relative evaporation rates be important to know about chemicals in terms of safety?
Attempt all these questions before we go through the answers:
Practice test:
|
| ||||
References:
Bodner, G. 2014. Colligative Properties. [online] Available at: http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch15/colligative.php[Accessed: 7 Feb 2014].
Teachnlearnchem.com, (2014). [online] Available at: http://www.teachnlearnchem.com/Vapor_2.jpg [Accessed 4 Dec. 2014].
Teachnlearnchem.com, (2014). [online] Available at: http://www.teachnlearnchem.com/Vapor_2.jpg [Accessed 4 Dec. 2014].
Chem.purdue.edu, (2014). Vapor Pressure. [online] Available at: https://www.chem.purdue.edu/gchelp/liquids/vpress.html [Accessed 5 Dec. 2014].
Chem1.com, (2014). [online] Available at: http://www.chem1.com/acad/webtext/solut/solut-images/raoultmech.png [Accessed 5 Dec. 2014].
Chem.purdue.edu. (2014). Boiling point elevation. [online] Retrieved from: http://www.chem.purdue.edu/gchelp/solutions/eboil.html [Accessed: 8 Feb 2014].
Chem.purdue.edu. (2014). Freezing point depression. [online] Retrieved from: http://www.chem.purdue.edu/gchelp/solutions/freeze.html [Accessed: 8 Feb 2014].
Goldbook.iupac.org. 2014. IUPAC Gold Book - concentration. [online] Available at: http://goldbook.iupac.org/C01222.html [Accessed: 7 Feb 2014].
Goldbook.iupac.org. 2014. IUPAC Gold Book - osmotic pressure, Π. [online] Available at: http://goldbook.iupac.org/O04344.html [Accessed: 7 Feb 2014].
Bodner, G. 2014. Colligative Properties. [online] Available at: http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch15/colligative.php[Accessed: 7 Feb 2014].
Teachnlearnchem.com, (2014). [online] Available at: http://www.teachnlearnchem.com/Vapor_2.jpg [Accessed 4 Dec. 2014].
Teachnlearnchem.com, (2014). [online] Available at: http://www.teachnlearnchem.com/Vapor_2.jpg [Accessed 4 Dec. 2014].
Chem.purdue.edu, (2014). Vapor Pressure. [online] Available at: https://www.chem.purdue.edu/gchelp/liquids/vpress.html [Accessed 5 Dec. 2014].
Chem1.com, (2014). [online] Available at: http://www.chem1.com/acad/webtext/solut/solut-images/raoultmech.png [Accessed 5 Dec. 2014].
Chem.purdue.edu. (2014). Boiling point elevation. [online] Retrieved from: http://www.chem.purdue.edu/gchelp/solutions/eboil.html [Accessed: 8 Feb 2014].
Chem.purdue.edu. (2014). Freezing point depression. [online] Retrieved from: http://www.chem.purdue.edu/gchelp/solutions/freeze.html [Accessed: 8 Feb 2014].
Goldbook.iupac.org. 2014. IUPAC Gold Book - concentration. [online] Available at: http://goldbook.iupac.org/C01222.html [Accessed: 7 Feb 2014].
Goldbook.iupac.org. 2014. IUPAC Gold Book - osmotic pressure, Π. [online] Available at: http://goldbook.iupac.org/O04344.html [Accessed: 7 Feb 2014].