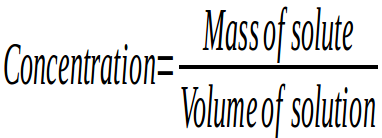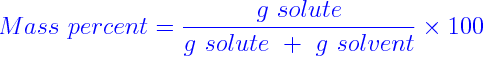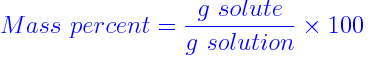UNIT 3 - PURE SUBSTANCES AND MIXTURES
Key concept - Systems - What systems can we use to observe and quanitfy what we see in our everyday life, and how can we communicate this information.
Related concepts - Form - How can we use scientific models to explain and predict these changes?
Global concept - Scientific and Technical Innovation - Matter is fundamental to our industrial and technological development, we need to understand its properties to utilise it to our best advantage.
Related concepts - Form - How can we use scientific models to explain and predict these changes?
Global concept - Scientific and Technical Innovation - Matter is fundamental to our industrial and technological development, we need to understand its properties to utilise it to our best advantage.
Key Words:
|
|
|
Task guide
The tasks and questions on the Weebly will be coloured to represent the different style of questions that you will find in your exams. The task should be completed in your "Natural Sciences" GoogleDrive document.
Green - Stating scientific knowledge
Orange - Applying scientific knowledge and understanding
Red - Analysing and evaluating information
There will also be "extension" tasks for students who finish tasks quickly! Also look out for links to interactive resources and videos.
The tasks and questions on the Weebly will be coloured to represent the different style of questions that you will find in your exams. The task should be completed in your "Natural Sciences" GoogleDrive document.
Green - Stating scientific knowledge
Orange - Applying scientific knowledge and understanding
Red - Analysing and evaluating information
There will also be "extension" tasks for students who finish tasks quickly! Also look out for links to interactive resources and videos.
Pure substances and mixtures
|
(ms deller science, 2017)
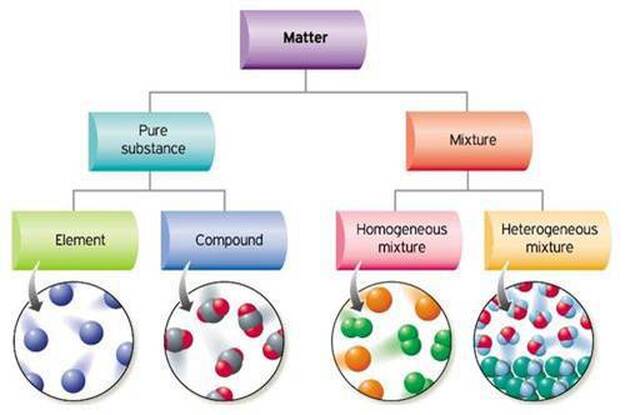
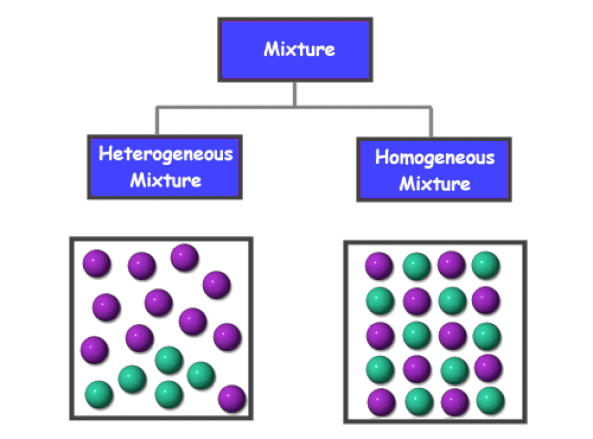
How are pure substances and mixtures different? Mixtures can be homogenous - this means that they are the same all the way through . For example, blood, air. Or they can be heterogenous - this means they are not the same all the way through,. You can see differences among its parts - they have 'lumps'. For example pizza. |
A pure substance always features the same composition and properties throughout (colour, point of fusion etc) and cannot be dissociated into simpler ones by simple physical methods. Water, iron, common salt, sulfuric acid and oxygen are just a few examples of pure substances.
Pure substances can be simple substances or compounds. Simple substances are made of just one type of atom; A piece of gold is made of only gold atoms. While compounds are made of more than one type of atom joined together; pure water or ammonia for example. On the other hand, mixtures contain more than one substance and have different composition and properties in different parts of the sample. For example, we know that air is made of Nitrogen - 78%, Oxygen - 20%, Argon, Carbon Dioxide and some other gases. Air is a mixture. of different gases. In a mixture, the substances are just mixed together and not chemically combined. |
Task 3a Here is a list of substances.:
Chocolate Carbon dioxide Soil Brass Orange Juice (with pulp) Carbon Coca Cola Steel Alcohol Iron
Hydrochloric Acid Oxygen Orange juice (without pulp) Pizza Magnesium Glucose Blood
Copy the table below and put the substances in the correct columns. Add 2 more substances of your own to each column.
Chocolate Carbon dioxide Soil Brass Orange Juice (with pulp) Carbon Coca Cola Steel Alcohol Iron
Hydrochloric Acid Oxygen Orange juice (without pulp) Pizza Magnesium Glucose Blood
Copy the table below and put the substances in the correct columns. Add 2 more substances of your own to each column.
|
A solution is a very special example of a homogenous mixture.
In a solution we have a solvent and a solute, The solute is the thing to be dissolved and the solvent is the thing it dissolves in.
For example if we have sugar in water - a sucrose solution, the solvent would be the water, the sucrose (sugar) would be the solute.
Solutions can be separated, if we have a solid disolved in liquid, by evaporating the liquid you will be left with the solid.
Separating other mixtures
All mixtures can be separated using the appropriate separation techniques. The separation technique used will depend on the properties of the components of the mixture.
Two solids of different size could be separated using a sieve. A mixture of two liquids can also be separated by decantation, if the liquids don't mix, such as water and oil. But if the liquids mix, such as water and alcohol, we would need to use a different method known as distillation.
Two solids of different size could be separated using a sieve. A mixture of two liquids can also be separated by decantation, if the liquids don't mix, such as water and oil. But if the liquids mix, such as water and alcohol, we would need to use a different method known as distillation.
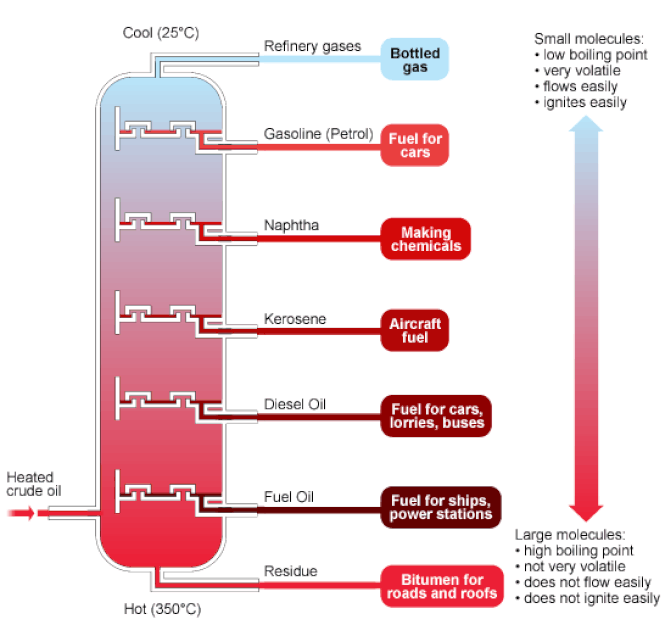
A good example of this is with crude oil, which looks like this: This in particular is fractional distillation.
You may recall from previous years yet another method, this time to separate a mixture of a solid that does not dissolve in a liquid, such as sand and water. Do you remember the name of that technique? Of course you do! Yes indeed, it is filtration.
Solution Concentration
Going back to our sucrose solution from above, we can say that our solution can be diluted or concentrated depending on the amount of solute (the sugar) and solvent (the water) that make up the solution. However, saying that our solution is diluted or very concentrated does not tell us much about the actual amounts of solute and solvent.
The concentration of a solution is the relationship between the amounts of solute and solvent. It is a measure of the quantity of solute in a given quantity of solvent or solution.
There are several ways to express the concentration of a solution. This year we will study two of those: Concentration by weight or Mass concentration, and Mass percent.
The concentration of a solution is the relationship between the amounts of solute and solvent. It is a measure of the quantity of solute in a given quantity of solvent or solution.
There are several ways to express the concentration of a solution. This year we will study two of those: Concentration by weight or Mass concentration, and Mass percent.
Concentration - calculations grams per litre (g/l)
The easiest way to imagine and quantify a solution is to ask two questions:
The solute can be liquid or gas, but for our purposes we can imagine it is a solid, so we measure its mass, in grams.
The only solvent that we will use this year to make our solution will be water,, so we measure its volume in litres.
So if we want to know how many grams of solute there are per litre of solution we use the formula:
- How much solute is there?
- How much solution do we have?
The solute can be liquid or gas, but for our purposes we can imagine it is a solid, so we measure its mass, in grams.
The only solvent that we will use this year to make our solution will be water,, so we measure its volume in litres.
So if we want to know how many grams of solute there are per litre of solution we use the formula:
Where: Mass is measured in grams, and volume is measured in litres, so concentration is measured in g/L
Example Calculations:
1. Find the concentration in g/L for 20 g of NaCl dissolved in 500 L of solution
Data Equation/Formula
20g NaCl
500 l solution mass concentration = g solute/L solution
mass conc. = ?
Calculations
mass concentration = 20g/500 L = 0.04 g/L
2. How many grams of bromine are needed to make 250 mL of a 4.5 g/L solution?
Data Equation/Formula
g Br= ?
250 mL solution mass conc. = g solute/L solution
mass conc. = 4.5 g/L so; g solute = (mass conc. )(L solution)
Calculations
g = 4.5/0.250 = 1.1 g Br
Data Equation/Formula
20g NaCl
500 l solution mass concentration = g solute/L solution
mass conc. = ?
Calculations
mass concentration = 20g/500 L = 0.04 g/L
2. How many grams of bromine are needed to make 250 mL of a 4.5 g/L solution?
Data Equation/Formula
g Br= ?
250 mL solution mass conc. = g solute/L solution
mass conc. = 4.5 g/L so; g solute = (mass conc. )(L solution)
Calculations
g = 4.5/0.250 = 1.1 g Br
| concentration_worksheet_2.pdf | |
| File Size: | 287 kb |
| File Type: | |
Concentration calculations - mass percent
another way to measure the concentration of a solution is the ratio of how much of the mass of the solution is provided by the solute.
If more of the mass is made up by the solute then its mass percent ratio will be higher.
This is given by the formula
If more of the mass is made up by the solute then its mass percent ratio will be higher.
This is given by the formula
Because the measurement of the mass is both in grams, they cancel and there are no units for this number, it is just a plain %age ratio.
As the mass of the solution is always the mass of the solute plus the mass of the solvent., we add them together to get the formula.. But don't forget that, as it is very important!
As the mass of the solution is always the mass of the solute plus the mass of the solvent., we add them together to get the formula.. But don't forget that, as it is very important!
We use 180 mL of pure water and 20g of alcohol to make a solution. What is the mass percent of alcohol of the solution?
Data Equation/Formula
180 mL of pure water = 180 g
20 g EtOH mass % =( g solute/ g solution) * 100
mass %. = ?
Calculations
mass % = 20/ (180 + 20) * 100 = 20/200 * 100 % = 10.0%
Data Equation/Formula
180 mL of pure water = 180 g
20 g EtOH mass % =( g solute/ g solution) * 100
mass %. = ?
Calculations
mass % = 20/ (180 + 20) * 100 = 20/200 * 100 % = 10.0%
Questions
1. What is the mass percent of NaCl (table salt) in a solution prepared by dissolving 450 grams of NaCl in 2420g of water?
2. What is the mass percent of a potassium iodide (KI) solution if it has 105g of KI(solute) in 400g of solution?
3. How many grams of solute are in 35g of a 12% salt solution?
4. I add 45g of table salt (NaCl) to prepare 120g of a salt solution, what is the mass percent of the solution?
5. If I get 500g of solute (KI) from a 60% solution, how many grams of solution did I have?
6. What is the mass percent concentration of a calcium chloride solution, if 578g of calcium chloride are dissolved in 3200g of water?
7. What is the mass percent concentration of a salt solution if 683 grams of salt are dissolved in water to make 950g of solution?
8. If I have a 37% zinc chloride solution, how many grams of zinc chloride are in 350g of solution?
9. I want to prepare a 70% NaCl solution. How many grams of salt do I need if I want 80g of solution?
1. What is the mass percent of NaCl (table salt) in a solution prepared by dissolving 450 grams of NaCl in 2420g of water?
2. What is the mass percent of a potassium iodide (KI) solution if it has 105g of KI(solute) in 400g of solution?
3. How many grams of solute are in 35g of a 12% salt solution?
4. I add 45g of table salt (NaCl) to prepare 120g of a salt solution, what is the mass percent of the solution?
5. If I get 500g of solute (KI) from a 60% solution, how many grams of solution did I have?
6. What is the mass percent concentration of a calcium chloride solution, if 578g of calcium chloride are dissolved in 3200g of water?
7. What is the mass percent concentration of a salt solution if 683 grams of salt are dissolved in water to make 950g of solution?
8. If I have a 37% zinc chloride solution, how many grams of zinc chloride are in 350g of solution?
9. I want to prepare a 70% NaCl solution. How many grams of salt do I need if I want 80g of solution?
Solubility
I am sure you have heard that term before. But what does it mean?
Let's go back once again to our sucrose solution. If we keep on adding sugar to our mixture, eventually the sugar will no longer dissolve in the water, but instead it will settle at the bottom of the glass. At this point we have saturated our solution
So, at that particular temperature no more sugar can be dissolved in that amount of water. The solution has reached its maximum capacity of solubility.
So we can define solubility as the maximum amount of solute that will dissolve in a solvent at a given temperature..
Let's go back once again to our sucrose solution. If we keep on adding sugar to our mixture, eventually the sugar will no longer dissolve in the water, but instead it will settle at the bottom of the glass. At this point we have saturated our solution
So, at that particular temperature no more sugar can be dissolved in that amount of water. The solution has reached its maximum capacity of solubility.
So we can define solubility as the maximum amount of solute that will dissolve in a solvent at a given temperature..
REFERENCES
Cdatacoinmdv.890m.com,. (2015). Result Of Factory Apprentice54 Batch | Cracked Data Coin Money. Retrieved 6 July 2015, from http://cdatacoinmdv.890m.com/result/result-of-factory-apprentice54-batch.html
Ducksters.com,. (2015). Chemistry for Kids: Chemical Mixtures. Retrieved 6 July 2015, from http://www.ducksters.com/science/chemistry/chemical_mixtures.php
Jones Oil Blog,. (2013). The Black Gold Rush: Where’s All the Oil? - Jones Oil Blog. Retrieved 6 July 2015, from http://www.jonesoil.ie/blog/the-black-gold-rush-wheres-all-the-oil/
SeaSalt Superstore, L. (2015). Salt History. Seasaltsuperstore.com. Retrieved 6 July 2015, from http://www.seasaltsuperstore.com/salt-history.aspx
Ducksters.com,. (2015). Chemistry for Kids: Chemical Mixtures. Retrieved 6 July 2015, from http://www.ducksters.com/science/chemistry/chemical_mixtures.php
Jones Oil Blog,. (2013). The Black Gold Rush: Where’s All the Oil? - Jones Oil Blog. Retrieved 6 July 2015, from http://www.jonesoil.ie/blog/the-black-gold-rush-wheres-all-the-oil/
SeaSalt Superstore, L. (2015). Salt History. Seasaltsuperstore.com. Retrieved 6 July 2015, from http://www.seasaltsuperstore.com/salt-history.aspx