When a liquid has not reached its boiling point, the vapour pressure is lower than the external pressure. This means the external pressure is able to keep the liquid in the liquid state.
The Atmosphere
What gases compose our atmosphere?
Has the atmospheric composition always been the same?
What is atmospheric pollution?
Has the atmospheric composition always been the same?
What is atmospheric pollution?
|
Keywords
|
|
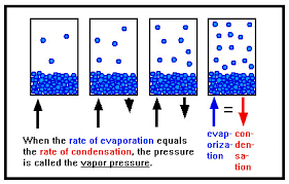
Vapour Pressure
The vapour pressure of a liquid is the pressure exerted by the vapour when equilibrium is reached between its vapour phase and its liquid phase.
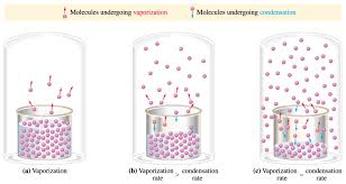
So, at any given temperature, for a particular substance there is a pressure at which the vapour of that substance is in equilibrium with its liquid form (the same number of particles leaving the liquid by evaporation return to the liquid by condensation). This is termed the vapour pressure of that substance at that temperature.
Why does this happen?
Well, as not all particles have the same kinetic energy, in all liquids at a given temperature, some particles will move faster than others. The faster ones and near the surface have enough energy to escape and turn into a gas – evaporation – As the number of these gas particles increases, so will the vapour pressure. Eventually a point will be reached where the inverse process will take place; gas particles will lose energy and will turn back into the liquid state – condensation - , reaching an equilibrium, when evaporation rate equals condensation rate. At this point the pressure exerted by the vapour is called the vapour pressure.
Why does this happen?
Well, as not all particles have the same kinetic energy, in all liquids at a given temperature, some particles will move faster than others. The faster ones and near the surface have enough energy to escape and turn into a gas – evaporation – As the number of these gas particles increases, so will the vapour pressure. Eventually a point will be reached where the inverse process will take place; gas particles will lose energy and will turn back into the liquid state – condensation - , reaching an equilibrium, when evaporation rate equals condensation rate. At this point the pressure exerted by the vapour is called the vapour pressure.
Boiling point
As temperature increases more molecules are able to escape the liquid, and as a consequence vapour pressure increases and vice versa. When the pressure reaches atmospheric pressure the entire liquid will start to boil. We can therefore say that the boiling point of a liquid is that at which its vapour pressure equals atmospheric pressure.
As temperature increases more molecules are able to escape the liquid, and as a consequence vapour pressure increases and vice versa. When the pressure reaches atmospheric pressure the entire liquid will start to boil. We can therefore say that the boiling point of a liquid is that at which its vapour pressure equals atmospheric pressure.
Vapor pressure of four common liquids, shown as a function of temperature |
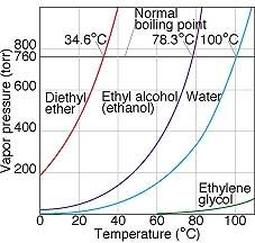
("Chapter 11, Section 5", 2018)
The Atmosphere
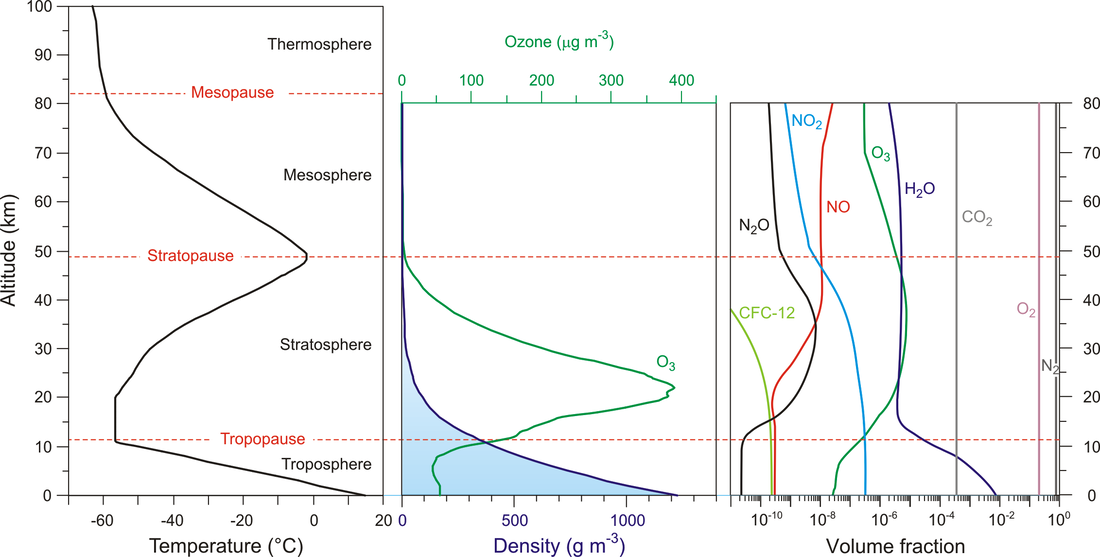
The atmosphere is the layer of gaeses that surrounds the Earth. It protects life on Earth by creating pressure allowing for liquid water to exist on the Earth's surface, absorbing UV Sun rays, maintaining the Earth warm by retaining the heat from our Sun (green house effect) and preventing temperature extremes between day and night.
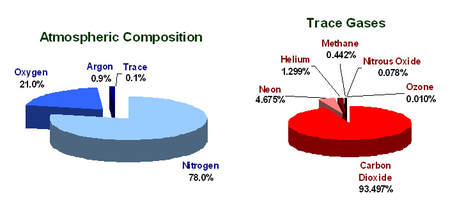
Atmospheric Composition
The Earth’s atmosphere is mainly composed of nitrogen (78%), oxygen (21%) and argon (1%). It also contains trace amounts of carbon dioxide, and other gases. At lower altitudes it also contains water vapour..
("North Carolina Climate Blog", 2018)
(Eni Generalic, 2018)
References:
Bodner, G. 2014. Colligative Properties. [online] Available at: http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch15/colligative.php[Accessed: 7 Feb 2014].
Teachnlearnchem.com, (2014). [online] Available at: http://www.teachnlearnchem.com/Vapor_2.jpg [Accessed 4 Dec. 2014].
Teachnlearnchem.com, (2014). [online] Available at: http://www.teachnlearnchem.com/Vapor_2.jpg [Accessed 4 Dec. 2014].
Chem.purdue.edu, (2014). Vapor Pressure. [online] Available at: https://www.chem.purdue.edu/gchelp/liquids/vpress.html [Accessed 5 Dec. 2014].
Chem1.com, (2014). [online] Available at: http://www.chem1.com/acad/webtext/solut/solut-images/raoultmech.png [Accessed 5 Dec. 2014].
Chem.purdue.edu. (2014). Boiling point elevation. [online] Retrieved from: http://www.chem.purdue.edu/gchelp/solutions/eboil.html [Accessed: 8 Feb 2014].
Chem.purdue.edu. (2014). Freezing point depression. [online] Retrieved from: http://www.chem.purdue.edu/gchelp/solutions/freeze.html [Accessed: 8 Feb 2014].
Goldbook.iupac.org. 2014. IUPAC Gold Book - concentration. [online] Available at: http://goldbook.iupac.org/C01222.html [Accessed: 7 Feb 2014].
Goldbook.iupac.org. 2014. IUPAC Gold Book - osmotic pressure, Π. [online] Available at: http://goldbook.iupac.org/O04344.html [Accessed: 7 Feb 2014].
Bodner, G. 2014. Colligative Properties. [online] Available at: http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch15/colligative.php[Accessed: 7 Feb 2014].
Teachnlearnchem.com, (2014). [online] Available at: http://www.teachnlearnchem.com/Vapor_2.jpg [Accessed 4 Dec. 2014].
Teachnlearnchem.com, (2014). [online] Available at: http://www.teachnlearnchem.com/Vapor_2.jpg [Accessed 4 Dec. 2014].
Chem.purdue.edu, (2014). Vapor Pressure. [online] Available at: https://www.chem.purdue.edu/gchelp/liquids/vpress.html [Accessed 5 Dec. 2014].
Chem1.com, (2014). [online] Available at: http://www.chem1.com/acad/webtext/solut/solut-images/raoultmech.png [Accessed 5 Dec. 2014].
Chem.purdue.edu. (2014). Boiling point elevation. [online] Retrieved from: http://www.chem.purdue.edu/gchelp/solutions/eboil.html [Accessed: 8 Feb 2014].
Chem.purdue.edu. (2014). Freezing point depression. [online] Retrieved from: http://www.chem.purdue.edu/gchelp/solutions/freeze.html [Accessed: 8 Feb 2014].
Goldbook.iupac.org. 2014. IUPAC Gold Book - concentration. [online] Available at: http://goldbook.iupac.org/C01222.html [Accessed: 7 Feb 2014].
Goldbook.iupac.org. 2014. IUPAC Gold Book - osmotic pressure, Π. [online] Available at: http://goldbook.iupac.org/O04344.html [Accessed: 7 Feb 2014].