Unit 5a - The Geosphere and the Atmosphere
Key concept - Systems - How do different environmental systems interact with each other on Earth?
Related concepts - Models and environment - How can we use scientific models to explain changes in our environment?
Global concept - Orientations in time and space - Why is the position of Earth in the solar system essential for our survival?
Related concepts - Models and environment - How can we use scientific models to explain changes in our environment?
Global concept - Orientations in time and space - Why is the position of Earth in the solar system essential for our survival?
Unit 2 KEYWORDS:
|
|
|
Our planetThe photo to the right is called "The Pale Blue Dot". It is a photo taken on the 14th February, 1990 by the Voyager 1 Space probe from 6 billion km away.
It shows the Earth in a scattered ray of light from the Sun as the space probe was leaving our solar system. Click on the photo to hear a famous quote by Carl Sagan. |
(Scharf, 2015)
|
What is the Earth made of?
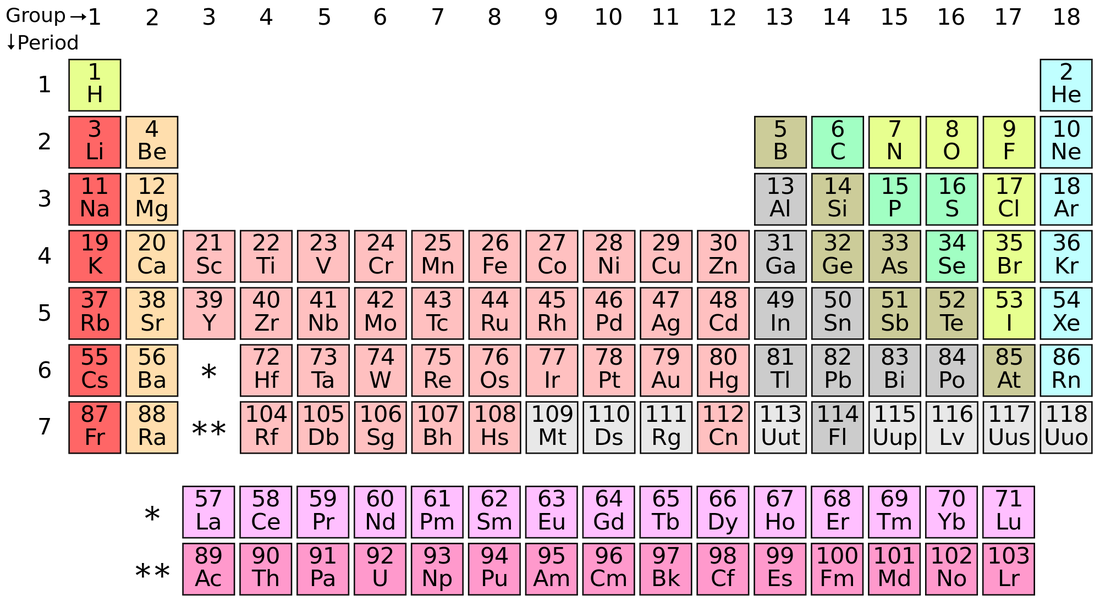
To fully understand how our Earth works, it is important to know the chemicals that make up the different systems. In science, we organise all of the chemicals into the periodic table (below). Each type of chemical has a name and a symbol.
|
(Lifestylewifi.com, 2015)
|
This table contains all of the chemical elements that we know. You will need to learn some of these names and symbols. The documents below contain the names and symbol that you need to learn. Download them and save them to your Natural Sciences document.
| ||||||||||||||
Task 2a: You will notice that some of the symbols are very different to the names of the elements. This is because the symbols are derived from the LATIN names not the ENGLISH ones.
Practice the names and symbols of the periodic table with this game, click here.
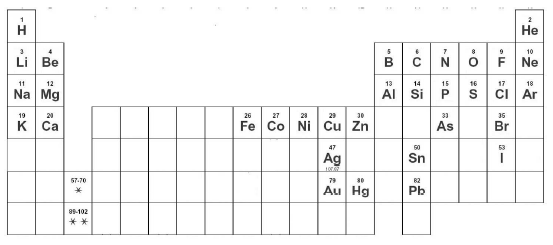
- Use the documents to write the names or symbols of these elements: F, Br, Na, Fe, H, chlorine, neon, potassium, oxygen and silver.
- Work out the English names from these Latin names and your knowledge of the symbols: cuprum, natrium, ferrum, stannum, kalium, argentum, aurum. Find the answers here.
- Chemical compounds are made from different types of element. Which elements do you think are in these chemical compounds: carbon dioxide, magnesium chloride, beryllium sulfide, water.
Practice the names and symbols of the periodic table with this game, click here.
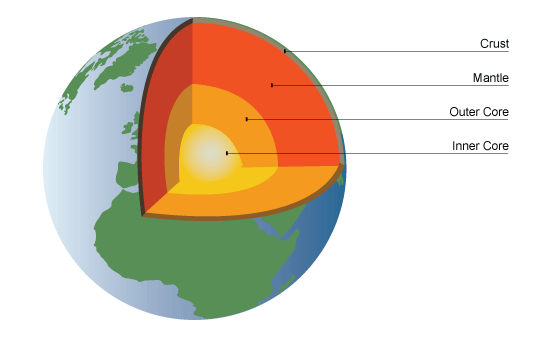
The lithosphere
|
Definition: The lithosphere is the rocky part of the Earth
The inner and outer core are formed mainly from the chemical elements iron (Fe) and nickel (Ni) and can have a temperature of up to 5,500 ºC. The mantle and the crust are solid and contain a large mixture of elements, including: Si, Al, Ca, Na, K and Mg. |
(Bbc.co.uk, 2015)
|
Task 2b:
- State which chemical elements have the symbols above?
- Find out which are the 2 most abundant (common) elements in the Earth´s crust?
- Although the mantle and crust are both solid, the mantle has a property that is more like a liquid. Which property is it?
What are minerals?
Definition: Minerals are naturally occurring substances with a definite chemical composition and a regular internal structure. Note: Most minerals are crystals, like salt and diamonds.
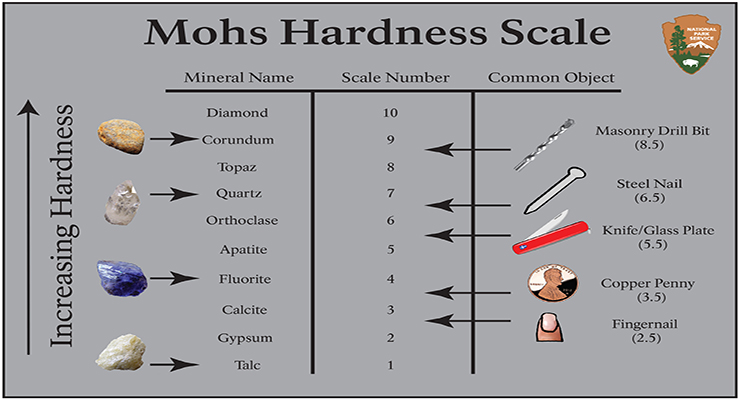
Friedrich Mohs
Believe it or not, in the early 1800’s no one had created a system for identifying minerals. So, Mr. Mohs started to study the differentphysical properties of mineralsas a way to identify them. He noticed that some minerals are very soft, others are extremely hard. He also observed that harder minerals could scratch softer ones.
With this in mind, Friedrich Mohs decided to develop a scratch test where he could determine the hardness of a mineral.To make his scratch test more accurate and helpful, he carefully observed many minerals and created a list of 10 minerals that he arranged from softest to hardest.
Believe it or not, in the early 1800’s no one had created a system for identifying minerals. So, Mr. Mohs started to study the differentphysical properties of mineralsas a way to identify them. He noticed that some minerals are very soft, others are extremely hard. He also observed that harder minerals could scratch softer ones.
With this in mind, Friedrich Mohs decided to develop a scratch test where he could determine the hardness of a mineral.To make his scratch test more accurate and helpful, he carefully observed many minerals and created a list of 10 minerals that he arranged from softest to hardest.
|
You will need to be able to describe certain properties of minerals:
Translucent - Light can pass through it but you cannot see objects through it. Opaque - No light can pass through it. |
(Fivestarstoneinc.com, 2014)
|
|
Task 2c:
|
|
(Stuffpoint.com, 2015)
|
RocksDefinition: A rock is a naturally occurring solid material containing 1 or more minerals.
There is a huge range of uses for rock that depend on their properties. You should be able to suggest uses according to the properties of a rock.
You will see the rock cycle in Unit 8. |
Task 2d:
Hints: Think about environmental, economic and human issues.
- Use the following interactive link to suggest which minerals are used in:
- Food production
- Construction
- Technology
- Food production
- A new type of rock has the following properties: is multi-coloured, shiny, easy to shape, resistant to rain. Suggest a use for this rock and explain your choice.
- The photo below shows the Bingham mine in America. It is the largest open mine in the world and is used to extract rocks containing copper (Cu). Evaluate the advantages and disadvantages of mining for rocks and minerals?
Hints: Think about environmental, economic and human issues.
(Wired UK, 2015)
|
Choose one of the following people and write a letter to the Salt Lake City Council explaining whether you think the mine should be closed or kept open:
The map to the right shows where the mine is and may help you decide how the mine would affect you. |
The atmosphere
Definition: The atmosphere is the gaseous part of the Earth
The Earth´s atmosphere is essential in providing the conditions for life on our planet. Without it, the average surface temperature would be -20 ºC and the amount of dangerous radiation from the Sun would cause large amounts of damage to living things. We would also have no oxygen to breathe!
The Earth´s atmosphere is essential in providing the conditions for life on our planet. Without it, the average surface temperature would be -20 ºC and the amount of dangerous radiation from the Sun would cause large amounts of damage to living things. We would also have no oxygen to breathe!
|
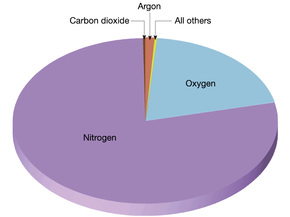
Task 2e: Read the text below and make a bar graph using the information in it:
It is clear from the pie chart that the main gas is nitrogen at 78 percent. Oxygen is the next most abundant gas at 21 percent. This is the gas that allows animals and plants to respire, and fuels to burn. All the other gases make up only 1 percent. These include carbon dioxide, water vapour and argon. (Bbc.co.uk, 2015)
|
All the gases in the atmosphere are made from chemical elements. Oxygen for example, has the chemical symbol O and nitrogen has the symbol N. Carbon dioxide actually contains one carbon (C) and two oxygens.
|
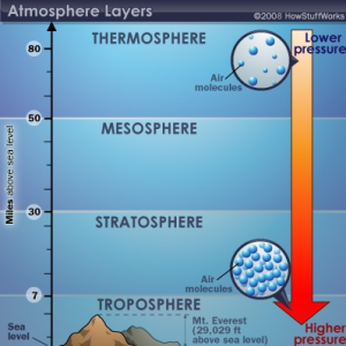
As the composition of the atmosphere is different in different places, scientists have named different layers. There are many layers that have been named, but the most common are the ones in this diagram.
You might also hear of other layers such as the ionosphere and the exosphere. Task 2f:
|
(Lamb, 2015)
|
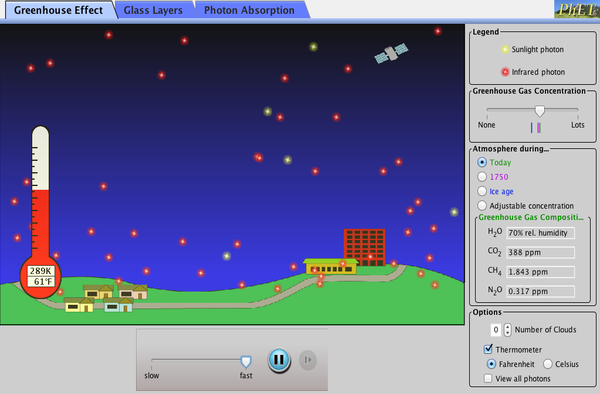
The Greenhouse Effect
|
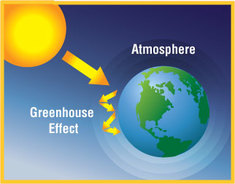
The most important role of the atmosphere is something called the greenhouse effect.
A greenhouse works by letting in the heat and light energy from the Sun but then preventing it from leaving again so that the temperature remains warm. The Earth´s atmosphere works in a similar way. It allows the Sun´s energy to pass through but then prevents most of it from escaping. Without the atmosphere, the energy would be lost back into space immediately. |
(Climatekids.nasa.gov, 2015)
|
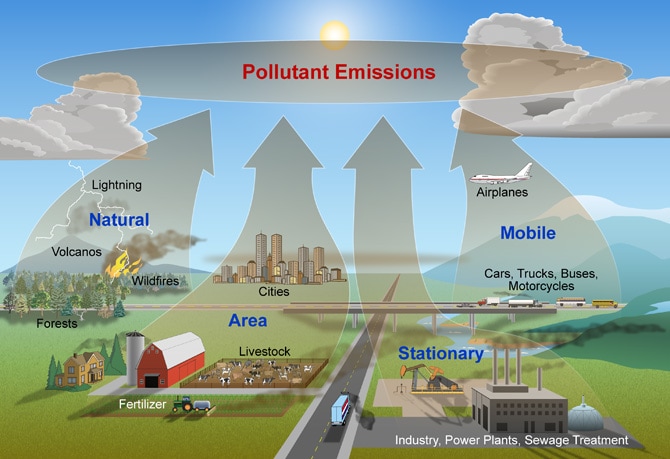
Atmospheric pollution
Atmospheric pollution can be difficult to see because the polluting substances are often invisible and come from many different places.. Air pollution happens when a substance that can cause negative effects to people or the ecosystem gets into the atmosphere.. According to the World Health Organisation, air pollution is responsible for the deaths of around 7 million people worldwide, every year.
Both human activity and natural processes can cause air pollution.
Some of the main pollutants are:
Some of the main pollutants are:
- Carbon Dioxide (CO2) - Because of its role as a greenhouse gas it has been described as "the leading pollutant" and "the worst climate pollution". Carbon dioxide is a natural part of the atmosphere, essential for plant life and given off by all animals. Recently, human activity has greatly increased the amount of C02 in the atmosphere.
- Sulpher oxides (SOx) - particularly sulfur dioxide (SO2). SO2 is produced by volcanoes and by burning coal and oil. It is the main cause of acid rain.
- Nitrogen oxides (NOx) - Nitrogen oxides, are given off by burning fossil fuels, and are also produced during thunderstorms by electric discharge. They can be seen as a brown haze above cities. and cause irritation to the lungs.
- Particulates also known as particulate matter (PM) - are tiny particles of solid or liquid suspended in the air. Some particulates occur naturally, from volcanoes, dust storms, forest and grassland fires, living vegetation, and sea spray. Human activities, such as the burning of fossil fuels in vehicles, power plants and various industrial processes also generate significant amounts.. Increased levels of fine particles in the air are linked to health hazards such as heart disease and lung cancer.
- Chlorofluorocarbons (CFCs) - These are gases which are released from air conditioners, refrigerators, aerosol sprays, etc. On release into the air, CFCs rise to the stratosphere. and damage the ozone layer. This allows harmful ultraviolet rays to reach the earth's surface. This can lead to skin cancer, eye disease and can even cause damage to plants.. After many years of work by environmentalists, CFCs are now banned in most countries.
- Odours - such as from garbage, sewage, and industrial processes
Task 2g
- State 3 ways that air pollution damages peoples health.
- Explain why human activity has increased the amount of atmospheric pollution.
Global warming
|
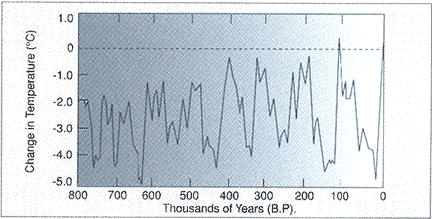
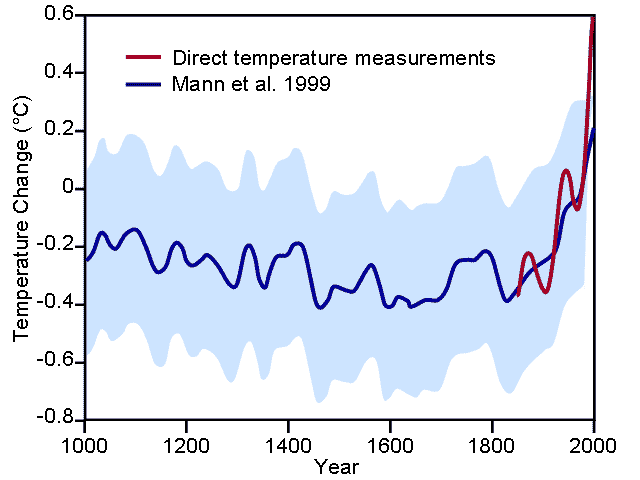
Over the million years, the average temperature of the Earth´s atmosphere has fluctuated (moved up and down) between cold periods and warm periods (right). Recently, however, human activities have started causing a particularly fast increase in the temperature of the Earth´s atmosphere (below). This increase in temperature is called. global warming.
|
(Geocraft.com, 2015)
|
|
(Savage, 2009)
|
Which human actvities do you know that have been causing these changes?
Three of the most important human activities that are causing global warming are:
We will focus on the burning of fossil fuels to provide energy. This releases large amounts of greenhouse gases, especially carbon dioxide, into the atmosphere. |
The more greenhouse gases that we release into the atmosphere, the more heat energy is trapped and this causes global warming.
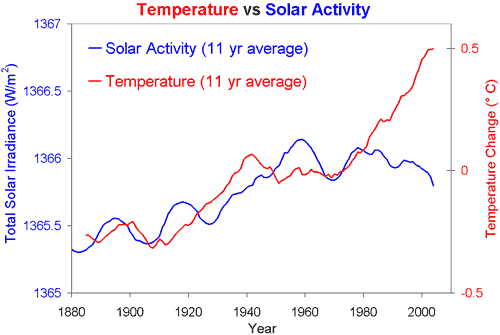
Extension: The amount of heat and light released by the Sun increases and decreases as we saw with the temperature of the Earth. Some scientist argue that this is the actual cause of global warming at the moment. Below is a graph that shows the temperature of the Earth and the energy released from the Sun ("solar activity") over the last 130 years. What do you think? Does the graph explain global warming? Explain your answer.
(Skeptical Science, 2015)
References:
ReferencesClimatekids.nasa.gov,. (2015). NASA's Climate Kids :: What is the greenhouse effect?. Retrieved 2 July 2015, from http://climatekids.nasa.gov/greenhouse-effect/
Fivestarstoneinc.com,. (2014). 3 Criteria To Consider When You Go For A Granite Countertop | Five Star Stone Inc Countertops. Retrieved 2 July 2015, from http://www.fivestarstoneinc.com/beautiful-granite-countertop/
Geocraft.com,. (2015). Global Warming:A Chilling Perspective. Retrieved 2 July 2015, from http://www.geocraft.com/WVFossils/ice_ages.html
Jones, J. (2011). Damien Hirst's skull tasteless? That's the point | Jonathan Jones. the Guardian. Retrieved 1 July 2015, from http://www.theguardian.com/artanddesign/jonathanjonesblog/2011/feb/22/damien-hirst-diamond-skull
News.bbc.co.uk,. (2015). BBC NEWS | Entertainment | Hirst's diamond skull raises �50m. Retrieved 2 July 2015, from http://news.bbc.co.uk/2/hi/entertainment/6971116.stm
Qyuhouse.website,. (2015). Greenhouse Effect Simple - Qyu House. Retrieved 2 July 2015, from http://qyuhouse.website/16306/greenhouse-effect-simple/
Savage, M. (2009). Life Sustaining Organizations: June 2009. Artofthefutureofwork.blogspot.com.es. Retrieved 2 July 2015, from http://artofthefutureofwork.blogspot.com.es/2009_06_01_archive.html
Skeptical Science,. (2015). IPCC Draft Report Leaked, Shows Global Warming is NOT Due to the Sun. Retrieved 3 July 2015, from http://skepticalscience.com/ipcc-draft-leak-global-warming-not-solar.html
Thehappyscientist.com,. (2015). Study Unit : Minerals Around You | The Happy Scientist. Retrieved 2 July 2015, from http://thehappyscientist.com/study-unit/minerals-around-you
Wired UK,. (2015). Bingham copper mine. Retrieved 2 July 2015, from http://www.wired.co.uk/news/archive/2013-04/22/earth-scars/viewgallery/303174
ReferencesClimatekids.nasa.gov,. (2015). NASA's Climate Kids :: What is the greenhouse effect?. Retrieved 2 July 2015, from http://climatekids.nasa.gov/greenhouse-effect/
Fivestarstoneinc.com,. (2014). 3 Criteria To Consider When You Go For A Granite Countertop | Five Star Stone Inc Countertops. Retrieved 2 July 2015, from http://www.fivestarstoneinc.com/beautiful-granite-countertop/
Geocraft.com,. (2015). Global Warming:A Chilling Perspective. Retrieved 2 July 2015, from http://www.geocraft.com/WVFossils/ice_ages.html
Jones, J. (2011). Damien Hirst's skull tasteless? That's the point | Jonathan Jones. the Guardian. Retrieved 1 July 2015, from http://www.theguardian.com/artanddesign/jonathanjonesblog/2011/feb/22/damien-hirst-diamond-skull
News.bbc.co.uk,. (2015). BBC NEWS | Entertainment | Hirst's diamond skull raises �50m. Retrieved 2 July 2015, from http://news.bbc.co.uk/2/hi/entertainment/6971116.stm
Qyuhouse.website,. (2015). Greenhouse Effect Simple - Qyu House. Retrieved 2 July 2015, from http://qyuhouse.website/16306/greenhouse-effect-simple/
Savage, M. (2009). Life Sustaining Organizations: June 2009. Artofthefutureofwork.blogspot.com.es. Retrieved 2 July 2015, from http://artofthefutureofwork.blogspot.com.es/2009_06_01_archive.html
Skeptical Science,. (2015). IPCC Draft Report Leaked, Shows Global Warming is NOT Due to the Sun. Retrieved 3 July 2015, from http://skepticalscience.com/ipcc-draft-leak-global-warming-not-solar.html
Thehappyscientist.com,. (2015). Study Unit : Minerals Around You | The Happy Scientist. Retrieved 2 July 2015, from http://thehappyscientist.com/study-unit/minerals-around-you
Wired UK,. (2015). Bingham copper mine. Retrieved 2 July 2015, from http://www.wired.co.uk/news/archive/2013-04/22/earth-scars/viewgallery/303174