Unit 1 - Scientific activity
Key concept - Systems - How do we quantify magnitudes in science?
Related concepts - Patterns and models - How can we use scientific conventions to better model the world around us?
Global concept - Scientific innovation and technology - How has science progressed in its ability to describe the physical world?
Related concepts - Patterns and models - How can we use scientific conventions to better model the world around us?
Global concept - Scientific innovation and technology - How has science progressed in its ability to describe the physical world?
|
Unit 1 KEYWORDS
|
|
|
|
Task guide
The tasks and questions on the Weebly will be coloured to represent the different style of questions that you will find in your exams. The task should be completed in your "Physics and Chemistry" GoogleDrive document (PSD).
Green - Explaining scientific knowledge
Orange - Applying scientific knowledge and understanding
Red - Analysing and evaluating information
There will also be "extension" tasks for students who finish tasks quickly! Also look out for links to interactive resources and videos.
The tasks and questions on the Weebly will be coloured to represent the different style of questions that you will find in your exams. The task should be completed in your "Physics and Chemistry" GoogleDrive document (PSD).
Green - Explaining scientific knowledge
Orange - Applying scientific knowledge and understanding
Red - Analysing and evaluating information
There will also be "extension" tasks for students who finish tasks quickly! Also look out for links to interactive resources and videos.
UNIT ANSWERS- LINK
1.1 Scalar magnitudes and vectors
|
Definition: Physical magnitudes are properties of objects that can be measured by comparison with a standard unit.
Note: A force of 5 N is not a vector unless a direction is given as well.
|
(Quenqua, 2012)
|
This content will be covered in greater detail in the 2nd and 3rd trimester.
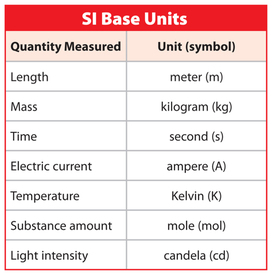
1.2 SI Units
Watch the following video that highlights some of the topics we will cover in this unit.
In 1960 the Système International d´Unités (SI) was agreed upon to enhance scientific communication.
The table on the left (Marshscience.blogspot.com.es, 2014) shows the 7 fundamental units agreed upon by this convention.
The table on the left (Marshscience.blogspot.com.es, 2014) shows the 7 fundamental units agreed upon by this convention.
Note: Symbols for units are always in lowercase unless the unit is named after somebody, in which case it is in UPPERCASE.
Derived units are units that contain a combination of fundamental units to describe quantities such as acceleration and electrical resistance.
1.3 Writing units
There are a number of conventions that we must follow when writing units:
- Leave a space between the number and symbol of the unit --> 25 kg NOT 25kg
- Units written as words are always lowercase --> 4 newtons NOT 4 Newtons
- Decimals are always written with a point/comma below --> 19.6 or 19,6 NOT 19`6
Task 1a: Identify which of the following units are written incorrectly and rewrite them. in your PCD.
Which other units exist for temperature?
What relevance do the 2 following objects have in this topic? Click on the photos to find out more.
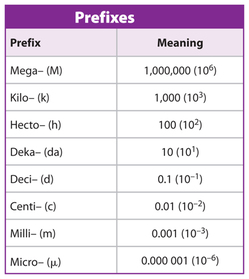
- 1000Mm
- 5 Candela
- 25.06 ccd
- 94 K
- 74888 m seconds
- 0.004Moles
- 20 hm
- 12 µ m
Which other units exist for temperature?
What relevance do the 2 following objects have in this topic? Click on the photos to find out more.
|
Lab session 1 - Paper balls
The first lab session will be related to the following content on making measurements and presenting data.
| ||||
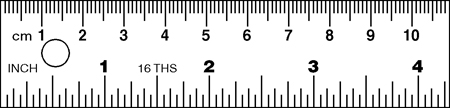
1.4 Making measurements - Precision
Precision: Which of these pieces of equipment is the most precise?
The answer is the measuring cylinder because it allows us to measure to the smallest division of the unit. The bowl allows us to measure to the nearest 500 ml, the beaker to the nearest 25 ml and the measuring cylinder to the nearest 1 ml. The precision would be written ± 500 ml, ± 25 ml and ± 1 ml respectively.
Task 1b:
- Find and paste 2 different images into your PCD showing equipment with different precision used to measure: time; mass and volume (with different equipment to the ones shown above!). Write the precision for each one.
- Which is the most precise piece of equipment in the chemistry lab?
- Write a procedure to make a 0.5 M solution of NaOH..
1.5 Making measurements - Error
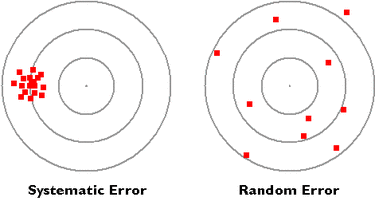
Experimental measurements will always be affected by error. The two types of error that can occur in an experiment are systematic and random.
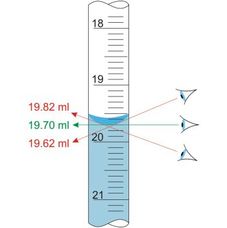
Systematic errors - arise from a problem in the experimental set-up that results in the measured values always deviating from the “true” value in the same direction, that is, always higher or always lower. For example, if a scale is incorrectly calibrated and always gives a slightly higher reading than it should (calibration error). We can also find systematic error in the incorrect reading of measurements as shown in the second diagram (parallax error). Systematic errors are difficult to identify as the error is always carried in the same direction so it is not obvious that it is occuring.
Random errors – are caused by unpredictable changes in the equipment or conditions of an experiment. They can lead to measurements being above or below the “true” value. A random change in the current flow in an electrical circuit would be an example. Random errors can be reduced with the use of more precise measuring equipment or its effect minimized through repeat measurements so that their effect is minimised when an average is taken.
An accurate result is a result that is close to the true value. This is done by minimising systematic and random errors.
Hint: When writing an evaluation of an experiment, look at each step in the procedure and consider the possibility of systematic and random error in each step.
Task 1c i: Make a list of 3 common random errors and 3 common systematic errors that could occur in an experiment.
Task 1c ii - Read the following procedure and explain all the possible systematic and random errors that could be present.
Preparing 500 ml of a 1 M solution of NaOH
Task 1c ii - Read the following procedure and explain all the possible systematic and random errors that could be present.
Preparing 500 ml of a 1 M solution of NaOH
- Weigh 20 g of NaOH powder in a weighing boat using a simple scale.
- Fill a large beaker with 500 ml distilled water.
- Add the NaOH to the water and stir for 30 seconds.
Identify any weaknesses in the evaluation and suggest at least 3 improvements that could be made.
1.6 Significant figures
Significant figures (sig figs) are the figures in a number that allow us to appreciate the precision in a measurement.
For example: In a measurement of 7.3 mm, which has 2 sig figs, the final sig fig tells us the unit division in which the error is found. In this case the last sig fig is the 3 (tenths of a millimeter) so the precision of the measurement is ± 0.1 mm.
For example: In a measurement of 7.3 mm, which has 2 sig figs, the final sig fig tells us the unit division in which the error is found. In this case the last sig fig is the 3 (tenths of a millimeter) so the precision of the measurement is ± 0.1 mm.
Rules for determining significant figures:
For the final rule, it can be difficult when given data that is not yours to appreciate the number of significant figures so we often represent the data using scientific notation...
- Figures that are not zero are always significant --> 194.78
- All figures to the right of the decimal point are significant (even zero) --> 23.40
- Zeros at the beginning of a number are not significant --> 0.00520 0.82
- Zeros at the end of a number without a decimal point are not significant --> 35400 105000300
For the final rule, it can be difficult when given data that is not yours to appreciate the number of significant figures so we often represent the data using scientific notation...
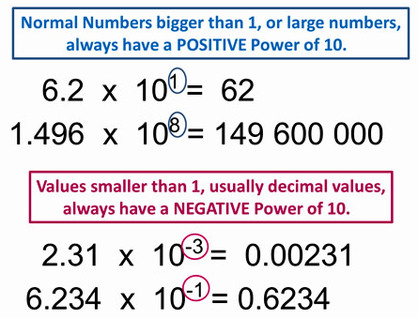
1.7 Scientific notation
When dealing with very small or very large numbers it is preferable to use scientific notation. This must be in a format with only 1 figure before the decimal point and then a positive or negative power of 10.
|
Task 1d: How many significant figures are found in the following numbers?
|
Write the following in scientific notation:
|
1.8 Using SFs and DPs in calculations
When carrying out calculations with experimentally retrieved data we must handle the number of sig figs/decimal places carefully to ensure we do not lose or gain unnecessary accuracy in the final result. There are 3 simple rules to follow.
1. If you are multiplying or dividing values then we consider the number of sig figs. The lowest number of significant figures used in the calculation should be given in the result.
2. If you are adding or subtracting values then we consider the number of decimal places. The lowest number of decimal places used in the calculation should be given in the result.
3. Numbers that are NOT from your data can be ignored when using the rules above. So when calculating an average from 3 numbers, you may ignore the `3´ that is used to divide them - it is an exact number.
Here are some examples:
4.80 + 2.60 + 1.2 = 8.6 (Lowest number of decimal places is 1)
3.5 x 19.36 = 68 (Lowest number of significant figures is 2)
Calculating an average: (1.68 + 8.52 + 7.31) / 3 = 5.84 (Lowest number of decimal places is 2 as the `3´ can be ignored)
1. If you are multiplying or dividing values then we consider the number of sig figs. The lowest number of significant figures used in the calculation should be given in the result.
2. If you are adding or subtracting values then we consider the number of decimal places. The lowest number of decimal places used in the calculation should be given in the result.
3. Numbers that are NOT from your data can be ignored when using the rules above. So when calculating an average from 3 numbers, you may ignore the `3´ that is used to divide them - it is an exact number.
Here are some examples:
4.80 + 2.60 + 1.2 = 8.6 (Lowest number of decimal places is 1)
3.5 x 19.36 = 68 (Lowest number of significant figures is 2)
Calculating an average: (1.68 + 8.52 + 7.31) / 3 = 5.84 (Lowest number of decimal places is 2 as the `3´ can be ignored)
Task 1e: Calculate the answers to these questions taking into account significant figures and decimal places. (Assume all numbers are experimentally obtained)
a. 5.8 + 4.56 + 12 =
b. 0.008 – 0.0456 + 0.9 =
c. 11 x 222 x 333 =
d. 19.30 / 182.3 =
e. (4.5 x 10^3) x (18.93 + 2.07) =
f. What is the average of these data points – 42.66, 43.810, 43.0
Complete the following online test: http://www.sciencegeek.net/Chemistry/taters/Unit0Sigfigs.htm
a. 5.8 + 4.56 + 12 =
b. 0.008 – 0.0456 + 0.9 =
c. 11 x 222 x 333 =
d. 19.30 / 182.3 =
e. (4.5 x 10^3) x (18.93 + 2.07) =
f. What is the average of these data points – 42.66, 43.810, 43.0
Complete the following online test: http://www.sciencegeek.net/Chemistry/taters/Unit0Sigfigs.htm
Extension:
- State the symbols for the following units: milliseconds, gigacandela, nanomol, microkelvin.
- Write the following in scientific notation: 450 0.0056 988300200 0.00000000014
- Identify the incorrectly written magnitudes and write them correctly: 42 ms, 78kg, 5.32 da cd, 1 cm/s.
1.9 Analysis of data
|
You will be required to represent data obtained in the lab in a graphical format. Normally a scattergraph with a line of best fit.
Task 1e: Use Microsft Excel to draw a scattergraph for ONE of the following simple tables of data. For each one include:
|
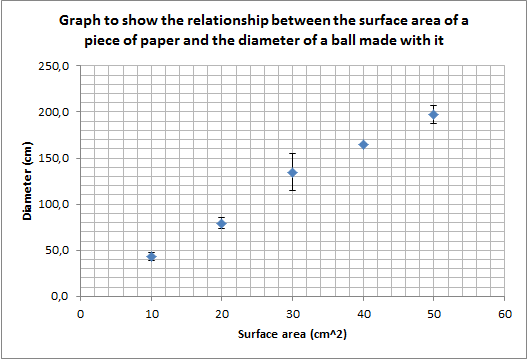
Task 1f: Dowload the following table of results from Mr Canning´s paper ball experiment and complete the following tasks:
When finished compare your graph with the answer on the second tab.
Help: Adding standard deviation and error bars
- Complete the mean (average) column and the SD (standard deviation) column
- Plot a scatter graph of the surface area v diameter (the mean).
- Add error bars using standard deviation.
When finished compare your graph with the answer on the second tab.
Help: Adding standard deviation and error bars
1.10 Conversion factors
Conversion factors are a very useful way of dealing with unit conversion and also many P+C problems. Watch the video below and try the following
|
Task 1g: Convert these values using conversion factors:
6 days into hours 12 mins into seconds 1825 days into years 700 dm into hm 3.7 km into cm 9 dam into mm 20 m/s into km/h 144 km/h into m/s 5 m/s into dm/min 6 hm/h into m/min |
Use the answers to check your accuracy!:
144 h 720 s 5 years 0.7 370000 90000 72 40 3000 10 |
Class presentation
|
Class presentation -->
| ||||
Revision
Practice exam questions
| |||
References:
220-3NM, L. (2015). Lab Balance Scale Kern EW 220-3NM. Labanalyticalbalance.com. Retrieved 23 July 2015, from http://www.labanalyticalbalance.com/lab-balance-scale-kern-ew-220-3nm.html
Amazon.com,. (2015). Graduated Cylinders: Amazon.com. Retrieved 22 July 2015, from http://www.amazon.com/b?node=393349011
Denovoverseas.com,. (2015). :::::Welcome to Denovo Overseas:::::. Retrieved 22 July 2015, from http://www.denovoverseas.com/bowls.html
E-education.psu.edu,. (2015). 5. Systematic vs. Random Errors | The Nature of Geographic Information. Retrieved 23 July 2015, from https://www.e-education.psu.edu/geog482spring2/c5_p5.html
EETimes,. (2015). Goodbye, Fundamental Kilogram & Ampere | EE Times. Retrieved 9 September 2015, from http://www.eetimes.com/author.asp?section_id=36&doc_id=1323806
Eni Generalic, C. (2015). Parallax @ Chemistry Dictionary & Glossary. Glossary.periodni.com. Retrieved 23 July 2015, from http://glossary.periodni.com/glossary.php?en=parallax
Fabricating and Metalworking,. (2013). What You Must Know About Calipers - Fabricating and Metalworking. Retrieved 22 July 2015, from http://www.fabricatingandmetalworking.com/2013/05/what-you-must-know-about-calipers/
Marshscience.blogspot.com.es,. (2014). Thomas C. Marsh Preparatory Middle School 7th Grade Science!: August 2014. Retrieved 22 July 2015, from http://marshscience.blogspot.com.es/2014_08_01_archive.html
Passy's World of Mathematics,. (2013). Scientific Notation. Retrieved 22 July 2015, from http://passyworldofmathematics.com/scientific-notation/
Quenqua, D. (2012). Wingsuits Let Jumpers Spread Their Wings. Nytimes.com. Retrieved 9 July 2015, from http://www.nytimes.com/2012/12/16/fashion/wingsuits-let-jumpers-spread-their-wings.html?_r=0
Sites.google.com,. (2015). Beaker - BioChem Group. Retrieved 22 July 2015, from https://sites.google.com/a/asu.edu/biochem-group/beaker
Spiff.rit.edu,. (2015). Retrieved 22 July 2015, from http://spiff.rit.edu/classes/phys200/lectures/ke_low/ke_low.html
Wholesalesafetypins.com,. (2015). Wholesale Prices on L-Squares, T-Squares, and Precision Rulers. Retrieved 22 July 2015, from http://wholesalesafetypins.com/straight-edge-rulers.html
220-3NM, L. (2015). Lab Balance Scale Kern EW 220-3NM. Labanalyticalbalance.com. Retrieved 23 July 2015, from http://www.labanalyticalbalance.com/lab-balance-scale-kern-ew-220-3nm.html
Amazon.com,. (2015). Graduated Cylinders: Amazon.com. Retrieved 22 July 2015, from http://www.amazon.com/b?node=393349011
Denovoverseas.com,. (2015). :::::Welcome to Denovo Overseas:::::. Retrieved 22 July 2015, from http://www.denovoverseas.com/bowls.html
E-education.psu.edu,. (2015). 5. Systematic vs. Random Errors | The Nature of Geographic Information. Retrieved 23 July 2015, from https://www.e-education.psu.edu/geog482spring2/c5_p5.html
EETimes,. (2015). Goodbye, Fundamental Kilogram & Ampere | EE Times. Retrieved 9 September 2015, from http://www.eetimes.com/author.asp?section_id=36&doc_id=1323806
Eni Generalic, C. (2015). Parallax @ Chemistry Dictionary & Glossary. Glossary.periodni.com. Retrieved 23 July 2015, from http://glossary.periodni.com/glossary.php?en=parallax
Fabricating and Metalworking,. (2013). What You Must Know About Calipers - Fabricating and Metalworking. Retrieved 22 July 2015, from http://www.fabricatingandmetalworking.com/2013/05/what-you-must-know-about-calipers/
Marshscience.blogspot.com.es,. (2014). Thomas C. Marsh Preparatory Middle School 7th Grade Science!: August 2014. Retrieved 22 July 2015, from http://marshscience.blogspot.com.es/2014_08_01_archive.html
Passy's World of Mathematics,. (2013). Scientific Notation. Retrieved 22 July 2015, from http://passyworldofmathematics.com/scientific-notation/
Quenqua, D. (2012). Wingsuits Let Jumpers Spread Their Wings. Nytimes.com. Retrieved 9 July 2015, from http://www.nytimes.com/2012/12/16/fashion/wingsuits-let-jumpers-spread-their-wings.html?_r=0
Sites.google.com,. (2015). Beaker - BioChem Group. Retrieved 22 July 2015, from https://sites.google.com/a/asu.edu/biochem-group/beaker
Spiff.rit.edu,. (2015). Retrieved 22 July 2015, from http://spiff.rit.edu/classes/phys200/lectures/ke_low/ke_low.html
Wholesalesafetypins.com,. (2015). Wholesale Prices on L-Squares, T-Squares, and Precision Rulers. Retrieved 22 July 2015, from http://wholesalesafetypins.com/straight-edge-rulers.html