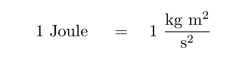Topic 1 - Stoichiometric relationships
Prior knowledge
|
|
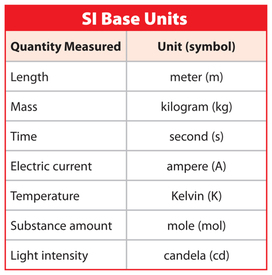
In 1960 the Système International d´Unités (SI) was agreed upon to enhance scientific communication. The table on the left (Marshscience.blogspot.com.es, 2014) shows the 7 fundamental units agreed upon by this convention. |
Note: Symbols for units are always in lowercase unless the unit is named after somebody, in which case it is in UPPERCASE.
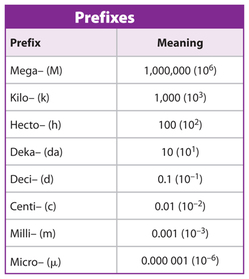
Derived units are units that contain a combination of fundamental units to describe quantities such as acceleration and electrical resistance.
There are a number of conventions that we must follow when writing units:
- Leave a space between the number and symbol of the unit --> 25 kg NOT 25kg
- Units written as words are always lowercase --> 4 newtons NOT 4 Newtons
- Decimals are always written with a point/comma below --> 19.6 or 19,6 NOT 19`6
Task 1a: Identify which of the following units are written incorrectly?
The photos below show what we used to use as the standard metre and kilogram:
- 1000Mm
- 5 Candela
- 25.06 ccd
- 94 K
- 74888 m seconds
- 0.004Moles
- 20 hm
- 12 µ m
The photos below show what we used to use as the standard metre and kilogram:
More recently (1984), the Geneva Conference on Weights and Measures has defined the meter as the distance light travels, in a vacuum, in 1/299,792,458 seconds with time measured by a cesium-133 atomic clock which emits pulses of radiation at very rapid, regular intervals.
Presentations
1.1 Presentation:
|
2015/16
|
2014/15
| ||||
1.2 presentation
|
1.3 Reacting masses and volumes
| ||
Questions
All of Topic 1
|
| ||||
1.1 The particulate nature of matter
1Balancing equations online practice - http://www.sciencegeek.net/Chemistry/taters/EquationBalancing.htm
1Balancing equations and formulation exercises:
|
| ||||
|
| ||||
1.2 The mole concept
|
| ||||
|
| ||||
1.3 Reacting masses and volumes
|
| ||||
|
| ||||
QR code quiz:
Selectividad questions
|
| ||||