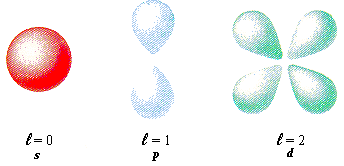Unit 4 - The Structure of Matter
|
Key concept - Relationships - How do observable properties relate to the structure of material?
Related concepts - Models and testing - How do models of scientific phenomena evolve and what importance does experimental testing have in this process? Global concept - Scientific innovation and technology - How has science progressed in its ability to describe the physical world? |
(Wwu.edu, 2015)
|
EXAM HINT - You will need to know all of the information on this page! (Class presentations are there as extra sources of information but may contain things that are not necessary for the exam)
Unit answers LINK
4.1 Atomic theories |
|
What is in an atom? - Subatomic particles
- Nucleus: The nucleus of the atom contains protons and neutrons
- Neutron: A neutron is a subatomic patticle contained in the atomic nucleus. It has no electric charge. The number of neutrons in an atomic nucleus determines the isotope of that element.
- Proton: A proton is a subatomic particle contained in the atomic nucleus. It has electric positive charge. The number of protons in an atomic nucleus determines the atomic number of that element and its nature.
- Electron: An electron is a subatomic particle found in the shell of an atom. It has electric negative charge. The number of electrons in an atom nucleus must equal the number of protons in the nucleus.
- Nucleus: The nucleus of the atom contains protons and neutrons
- Neutron: A neutron is a subatomic patticle contained in the atomic nucleus. It has no electric charge. The number of neutrons in an atomic nucleus determines the isotope of that element.
- Proton: A proton is a subatomic particle contained in the atomic nucleus. It has electric positive charge. The number of protons in an atomic nucleus determines the atomic number of that element and its nature.
- Electron: An electron is a subatomic particle found in the shell of an atom. It has electric negative charge. The number of electrons in an atom nucleus must equal the number of protons in the nucleus.
|
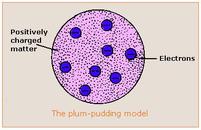
4.1b - Thomson: Thompson came up with the "plum pudding model" for the atom. He was the first scientist to propose that the atom was not the smallest particle but in fact contained small negative particles called electrons. He proposed that these electrons sat in a positive jelly like plums in a plum pudding.
|
|
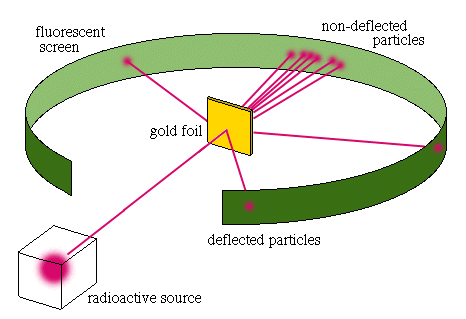
4.1c - Rutherford: Ernest Rutherford fired alpha particles (tiny positively charged particles) at a thin sheet of gold foil and found out that although most alpha particles went straight through the sheet, some were deflected or even reflected back towards their source.
Key observations:
|
Key conclusions:
Video - Rutherford experiment
Problem:
- The gold atoms must consist mostly of empty space as most particles passed through without hitting anything,
- That at the centre of the atom is a very small, dense region of positive charge that caused occasional deflection and even reflection. He called this the nucleus.
- Electrons must be moving freely in the empty space around the nucleus.
Video - Rutherford experiment
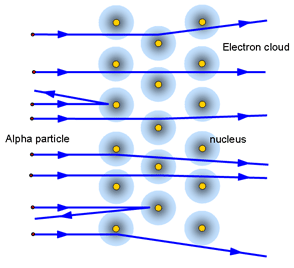
Problem:
- Classical physics suggested the electrons would lose energy as they moved freely around the nucleus and therefore should spiral into the nucleus.
- This model could not explain the lines found in atomic structure.
|
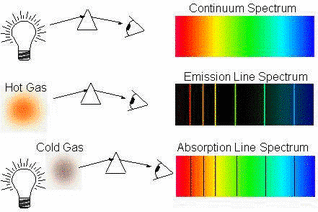
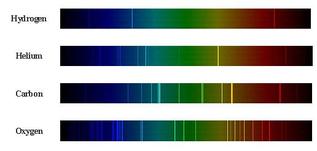
Different types of spectra
|
Passing normal white light through a prism (refraction) shows the full range of colours that it contains it a continuous spectrum.
If a gas is excited, electrons can jump up to a higher energy level (by absorbing energy) and then release that same amount of energy when they drop back down. Passing this light through a prism shows only the frequencies of light that are released by the drops. We call this an emmision spectrum. If, instead of exciting the gas, we shine white light through it then we see the opposite. We can see black lines where specific frequencies of light are being absorbed by electrons that are jumping up to higher energy levels. These are called absorption spectra. |
How did he use information from atomic spectra?
As these lines are only found at specific frequencies, it seems logical that they are produced by jumps or drops between specific energy levels. Therefore, not all energies are allowed.
The 3 postulates of Bohr’s atomic model.
Video - Bohr's model
As these lines are only found at specific frequencies, it seems logical that they are produced by jumps or drops between specific energy levels. Therefore, not all energies are allowed.
The 3 postulates of Bohr’s atomic model.
- Electrons orbit around the nucleus without losing energy.
- Only some orbits are permitted.
- Electrons change of orbit by absorbing or emitting the difference of energy between the initial and final level.
Video - Bohr's model
Haz clic aquí para modificar.
|
The existence of these spectra supports Bohr´s theory as only specific frequencies of light are seen. As the frequency of light is related to its energy then we must only have energy levels with a specific amount of energy. Therefore the electrons cannot move freely around the nucleus. As we can see below, the more electrons in an atom, the more lines we will see on its spectra:
|
The 3 postulates of Bohr’s atomic model:
- Electrons orbit around the nucleus without losing energy.
- Only some orbits are permitted.
- Electrons change of orbit by absorbing or emitting the difference of energy between the initial and final level.
This process can be used to determine the composition of stars that are many light years away as each element has a unique emission and absorption spectrum.
4.1e - Atomic orbital theory
Task 4a:
1. State the 3 postulates (conclusions) of Bohr´s model.
2. Describe the results of Rutherford´s model.
3. What is the "plum pudding" model?
4. What was the major difference between Dalton´s proposal and Thomson´s plum pudding model?
5. Why did Rutherford propose that the nucleus was very small?
6. What would be the main difference in atomic spectra given by a very small atom or a very large one?
7. How does Bohr´s model support the existence of atomic emission and absorption spectra?
8. Explain 2 problems with Rutherford´s model.
9. If Rutherford had carried out his experiment and found that all radioactive alpha particles were reflected, what might he have concluded?
10. If all atomic spectra had only 1 line at the same frequency of light, then what could Bohr have proposed about the structure of the atom?
1. State the 3 postulates (conclusions) of Bohr´s model.
2. Describe the results of Rutherford´s model.
3. What is the "plum pudding" model?
4. What was the major difference between Dalton´s proposal and Thomson´s plum pudding model?
5. Why did Rutherford propose that the nucleus was very small?
6. What would be the main difference in atomic spectra given by a very small atom or a very large one?
7. How does Bohr´s model support the existence of atomic emission and absorption spectra?
8. Explain 2 problems with Rutherford´s model.
9. If Rutherford had carried out his experiment and found that all radioactive alpha particles were reflected, what might he have concluded?
10. If all atomic spectra had only 1 line at the same frequency of light, then what could Bohr have proposed about the structure of the atom?
In 1924, Louis de Broglie suggested that electrons can actually behave like particles and waves - "wave-particle duality".
|
In accordance to wave-particle duality, in 1927, Werner Heisenberg came up with his uncertainty principle. This stated that we cannot know both the position and momentum of an electron at the same time (click on Walter White for a video on uncertainty).
This means that we cannot possibly describe the position of electrons in shells as Bohr suggested because they show an exact orbit where the electrons are. |
|
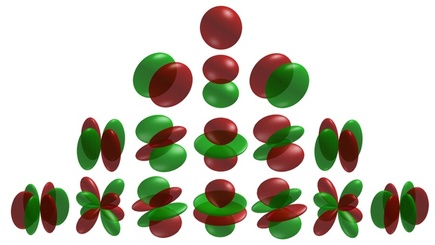
To include this principle in atomic orbital theory, a complex mathematical equation (The Schrödinger Equation) was used to predict areas in the atom where there was a 99% probability of finding an electron. These probability calculations show that depending on the energy level, there are a number of different areas (orbitals) in which the electron could be. The full range of orbitals is shown below. (You do not need to remember all of the shapes and names!!!) (Jha, 2013)
Each of the orbitals is capable of containing 2 electrons. |
Exension reading: One of the problems with Bohr´s atomic model was that the classical model of physics stated that the electron would be pulled into the nucleus. Read the article below to explain why the uncertainty priniple is important in solving this problem..
Read the following Guardian article (Jha, 2013).
Read the following Guardian article (Jha, 2013).
Task 4b:
1. For each of the "shells" (energy levels) that you have seen before, there are a particular number of orbitals (above) that are possible. Find a 3d diagram for the shapes of:
1. For each of the "shells" (energy levels) that you have seen before, there are a particular number of orbitals (above) that are possible. Find a 3d diagram for the shapes of:
- An s-orbital
- The three types of p-orbital
- The five types of d-orbital
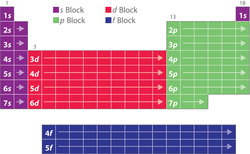
4.2 Electron configurations
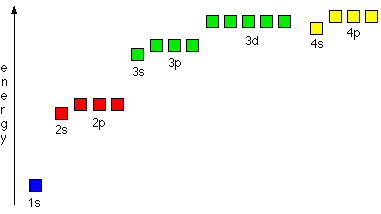
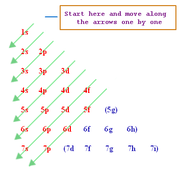
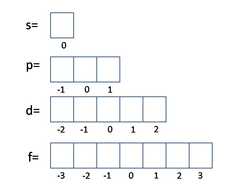
Using Heisenburg´s uncertainty principle, we find that in each energy level (as demonstrated by lines on atomic spectra) can be sub-divided into a number of atomic orbitals. The orbitals in each level are explained below left and the energies of these orbitals is shown below right. (Chemguide.co.uk, 2015)
If we know the number of electrons in an atom or ion then we can work out which energy level and orbitals that they are in by following 3 simple rules:
The Aufbau Principle - We must always fill up from the lowest energy orbitals first with 2 electrons in each.
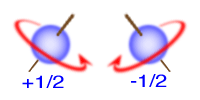
The Pauli Exclusion Principle - 2 electrons in the same orbital must be drawn with opposite spins.
Hund´s Rule - For orbitals with an equal energy we must place one electron in each before adding a second electron.
Animation on electrons filling in orbitals following aufbau rules. Click here.
Download the document below to see examples of electron configurations and how they are written.
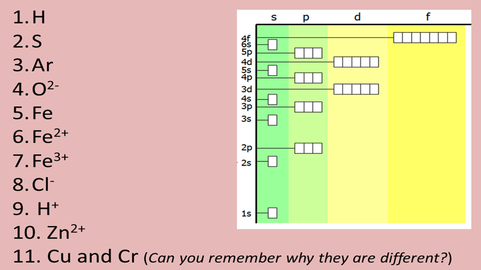
Task 4c: a. Complete the electron configurations for these atoms and ions:
This PowerPoint below may be useful in guiding you through the process:
|
| ||||
b.- extention: Consider the following electron configurations and explain why they might be the most common ions for each element:
- Sc 3+
- Fe 3+
- Cu +
- Cr 6+
- V 5+
|
SHORTCUTS!!!
The diagram to the right shows the patterns of how the orbitals are arranged in terms of energy. You will notice that each main energy level has one extra type of orbitals. When these have been written in order then you can see the pattern of the orbital energies. This is an easy way to write the e- configuration for any element in the periodic table! (Mpcfaculty.net, 2015) Just remember the number of electrons in each group of orbitals:
s --> 2 p --> 6 d --> 10 f --> 14 |
We can also use the electron configuration of any noble gases to replace the initial part of an electron configurations. For example:
Calcium - 20 electrons
Ca - 1s2 2s2 2p6 3s2 3p6 4s2
As the electron configuration of argon is - 1s2 2s2 2p6 3s2 3p6
We can state the electron configuration as:
[Ar] 4s2
Calcium - 20 electrons
Ca - 1s2 2s2 2p6 3s2 3p6 4s2
As the electron configuration of argon is - 1s2 2s2 2p6 3s2 3p6
We can state the electron configuration as:
[Ar] 4s2
Extension: Explain when we might find electrons in the g, h and i orbitals and explain why this would be difficult to do. This article may help you - "Are g-orbitals possible?"
4.3 Quantum numbers
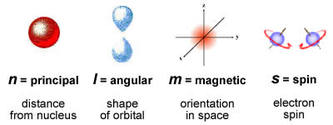
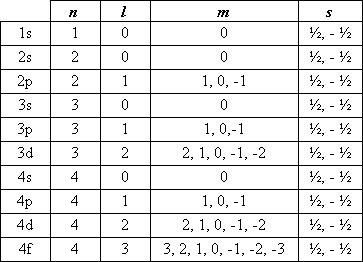
Each electron can be individually identified by a set of 4 numbers describing exactly where it is found. These are called quantum numbers.
n = Describes the main energy level in which the electron is found.
- An electron in the 1st energy level --> n = 1
- An electron in the 2nd energy level --> n = 2
- An electron in the 3rd energy level --> n = 3
|
m = This describes which specific orbital the electron is in. (left)
|
|
Summary
<-- This table summarises the possible quantum numbers in the first 4 energy levels. Task 4f:
|
Extension:
a. How many sets of quantum numbers could be associated with an electron in a 3p orbital?
b. Which properties of an element are affected by the "spin" of electrons? Why is it useful to know if we have paired and unpaired electrons in an atom? (https://www.youtube.com/watch?v=Isd9IEnR4bw#t=265)
a. How many sets of quantum numbers could be associated with an electron in a 3p orbital?
b. Which properties of an element are affected by the "spin" of electrons? Why is it useful to know if we have paired and unpaired electrons in an atom? (https://www.youtube.com/watch?v=Isd9IEnR4bw#t=265)
Task 4g:
Practice quizzes --> Medium quiz LINK Hard quiz: LINK
Practice exam style questions: https://docs.google.com/document/d/1h6yEF8Cy1LfpEVDWNuuAQo67s1YRgdw_D_gfuLleFbs/edit?usp=sharing
Practice quizzes --> Medium quiz LINK Hard quiz: LINK
Practice exam style questions: https://docs.google.com/document/d/1h6yEF8Cy1LfpEVDWNuuAQo67s1YRgdw_D_gfuLleFbs/edit?usp=sharing
|
4.1, 4.2, 4.3 Class presentation:
| |||
|
Practice exam questions -->
|
| ||
4.4 Atoms and the periodic table
|
Background information on atoms -->
|
| ||
|
Task 4h: Download this document and complete each slide using any available information.
|
| ||
The periodic table is organised by atomic number (the nunber of protons) and also arranged to place elements that share similar chemical properties in similar areas (normally groups(.
Groups are the column numbers (from 1-18) and periods are the horizonal rows. (from 1-7)
Groups are the column numbers (from 1-18) and periods are the horizonal rows. (from 1-7)
|
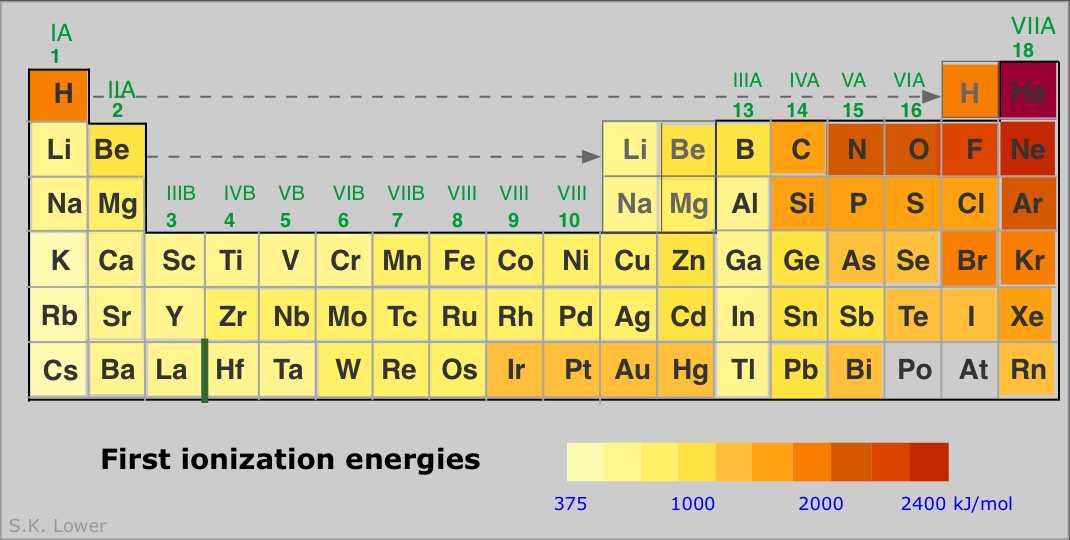
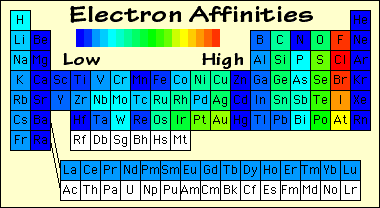
Periodicity is the word used to describe the patterns in the periodic table. We need to know the patterns in atomic radius (the size of atoms), electronegativity, ionisation energy and electron affinity.
a. Atomic radius Down a group - The atomic radius increases as there are more electrons, so more shells and more shielding from the nuclear charge.. Across a period - The atomic radius decreases as there are more protons (positive charge) in the nucleus but the electrons are all in the same shell so they have no extra shielding. The increase in positive charge means the outer electrons are pull more strongly into the nucleus.. |
|
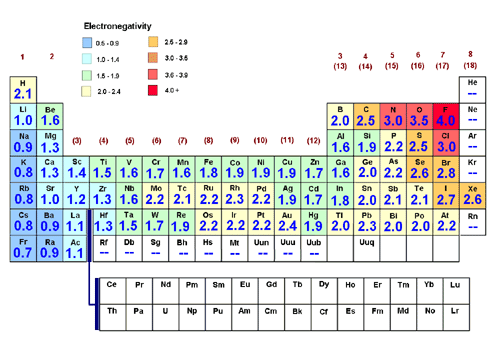
b. Electronegativity
Electronegativity is the ability of an atom to attract a bonding pair of electrons. (We measure it using the Pauling scale. In this scale fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least electronegative at 0.7). |
Down a group - The electronegativity decreases (as the bonding electrons feel less pull from the nucleus due to increased atomic radius and more shielding from the nuclear charge.).
Across a period - The electronegativity increases (as the bonding electrons are closer to the nucleus and feels more pull towards the nucleus).
Across a period - The electronegativity increases (as the bonding electrons are closer to the nucleus and feels more pull towards the nucleus).
|
(Chemwiki.ucdavis.edu, 2015)
|
c. Ionisation energy
If we remove an electron from an atom, we make a positive ion: F --> F+ + e- The energy required to do this is called the ionisation energy. If an element has a high electronegativity value, this means there is a strong attraction between the nucleus and the surrounding electrons. |
We see a strong correlation between the electronegativity values and the first ionisation energies because if an element pulls more strongly on their electrons, it will require more energy to remove them.
|
d. Electron affinity
Adding an electron to an atom creates a negative ion: Cl + e- --> Cl- Because of the attraction between the positive nucleus and negative electron, this process releases energy. As with ionisation energies, the most electronegative elements have the strongest attraction on electrons and release more energy when they gain one. |
Note: The correlation is more complicated than the others but you only need to know the general trends.
How is electronegativity related to types of bonding?
Covalent bonding occurs if...
2 atoms are equally electronegative and therefore both have the same tendency to attract the bonding pair of electrons. This means they would share the electrons. Normally we say a compound contains covalent bonding if the difference in electronegativity is less than 1.8. If 2 atoms have equal electronegativity then we would describe the bonding as pure covalent bonding and if there is a small difference then we would call it a polar covalent bond. E.g. Cl2 and CH4.
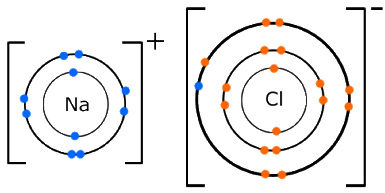
Ionic bonding occurs if...
2 atoms are have a large difference (more than 1.8) in electronegativity. This means that 1 atom would completely remove an electron from the other. E.g LiCl.
Metallic bonding occurs if...
We have only metal atoms present.
2 atoms are equally electronegative and therefore both have the same tendency to attract the bonding pair of electrons. This means they would share the electrons. Normally we say a compound contains covalent bonding if the difference in electronegativity is less than 1.8. If 2 atoms have equal electronegativity then we would describe the bonding as pure covalent bonding and if there is a small difference then we would call it a polar covalent bond. E.g. Cl2 and CH4.
Ionic bonding occurs if...
2 atoms are have a large difference (more than 1.8) in electronegativity. This means that 1 atom would completely remove an electron from the other. E.g LiCl.
Metallic bonding occurs if...
We have only metal atoms present.
4.5 Types of bonding
4.5 Class presentation
| |||
|
Ionic bonding
One atom completely removes one or more electrons from another to form charged ions with full outer shells. The actual ionic bond is the electrostatic attraction between the positive and negative charges. Properties - High melting and boiling points; dissolve in water but not organic solvents; and conduct electricity when dissolved in water but not when solid. |
|
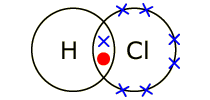
Covalent bonding
Atoms share electrons to complete their outer shell. The actual covalent bond is due to the attraction between the pair of shared electrons and the 2 nuclei. Properties - Low melting and boiling points; do not dissolve in water but DO dissolve in organic solvents; and do not conduct electricity. |
If we look more closely at covalent compounds we can further separate them into 2 types:
|
Simple covalent molecules
Molecules that contain covalent bonds between atoms in each molecule but only intermolecular forces between molecules. Examples:.
|
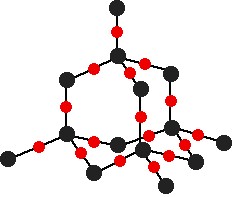
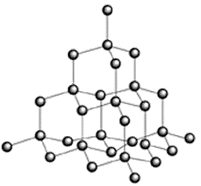
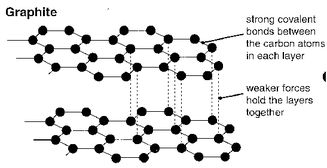
Giant covalent structures (lattices)
Structures where every atom in the structure is covalently bonded to another atoms. Important examples:
|
|
Properties - Low melting and boiling points; do not dissolve in water but DO dissolve in organic solvents (like acetone); and do not conduct electricity.
|
Properties - VERY high melting points; do not dissolve in water or organic solvents (like acetone).
Why such high melting points? This is because melting a giant covalent structure would require breaking all the strong covalent bonds. Giant covalent structures also do not dissolve in water or in organic solvents such as hexane. |
|
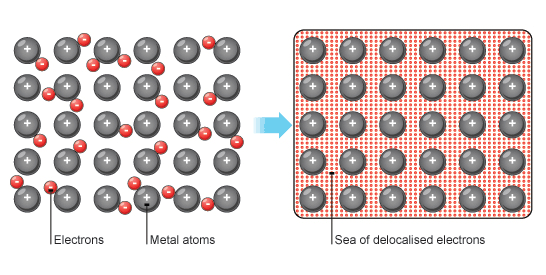
Metallic bonding
Metal atoms lose their outer electrons to form a structure of positive metal ions and a sea of delocalised electrons. The metallic bond is the strong attraction between the metal ions and the electrons. Properties - Very high melting and boiling points; do not dissolve in water or in organic solvents; and do conduct electricity. The ability of electrons to move freely also means that it is an excellent conductor of heat! |
|
Task 4i:
1. A substance has a low melting point and dissolves in organic solvents. What type of bonding is it likely to have? 2. Why do ionic compounds conduct electricity when dissolved in water but not when solid? 3. a. Why do we make frying pans using metallic substances? b. Why don´t we use simple covalent substances to make frying pans? 4. Diamond (above left) and graphite (left) are both giant covalent structures made only of carbon. Why is diamond one of the hardest substances we know yet graphite can be used in pencils? 5. Why is the radius of an Mg2+ ion considerably smaller than a Mg atom? 6. Compare and explain the radius of a Cl atoms and a Cl- ion. |
Task 4j: Create an infographic on the types of bonding and the properties associated with each one.
Examples of infographic websites: popplet, easel.ly, canva
Extension: Explain why are the electronegativity values of the noble gases usually ignored?
Examples of infographic websites: popplet, easel.ly, canva
Extension: Explain why are the electronegativity values of the noble gases usually ignored?
4.6 Intermolecular forces (of attraction)
|
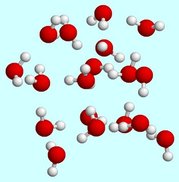
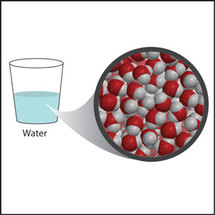
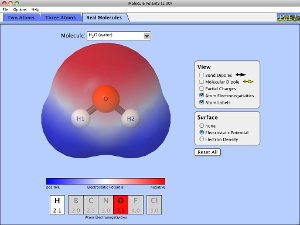
If we look closely at the H2O molecules in a glass of water you might notice that although we can see the oxygen and hydrogen atoms bonded together, we cannot see anything between the different molecules.
So why do the water molecules stay together in the glass? You will find the answer in the following section. |
|
Task 4k: Watch the video above and then complete the following document about the 3 types of intermolecular forces -->
|
| ||
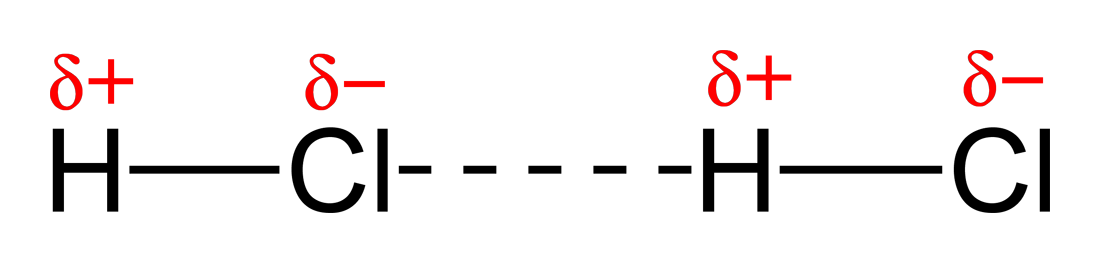
Intermolecular forces (of attraction) are weak forces that occur between molecules. There are 3 types:
Helpful LINK
Helpful LINK
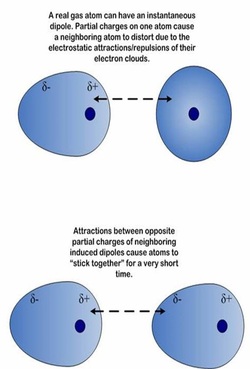
London forces
- The weakest IMF
- The random movement of electrons in molecules causes temporary dipoles
- These can "induce" (cause) a temporary dipole in another molecule.
- The 2 temporary dipoles attract for a very short period of time..
- Occurs in all molecules.
|
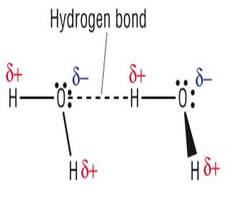
Hydrogen bonding
|
|
Task 4L
1. Which types of IMF´s are found in CH4, H2O, HCl and Br2? 2. Why do we represent IMF´s as dotted lines and not solid lines? 3. HCl and Ar are similar sizes but HCl has a much higher boiling point. Explain why? 4. Use the polarity simulation to the left to explain why boron trifluoride (BF3), carbon tetrafluoromethane (CF4) and carbon dioxide (CO2) are not described as polar molecule. |
Extension: Explain how IMF´s explain the phenomena shown in each image below:
Why are we interested in IMF´s?
Intermolecular forces can be used to explain many physical properties - melting and boiling points, surface tension, evaporation rate, viscosity and vapor pressure.
Examples:
1. A substance with strong intermolecular forces will have a higher melting and boiling point as the molecules will require more energy to overcome the IMF´s when becoming a liquid or a gas.
Examples:
1. A substance with strong intermolecular forces will have a higher melting and boiling point as the molecules will require more energy to overcome the IMF´s when becoming a liquid or a gas.
|
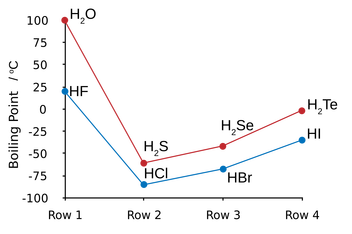
Both lines represent similar chemical substances however both H2O and HF do not follow the trend as they both have hydrogen bonding which means far more energy is required to change them into a gas.
The following molecules on each line then increase in boiling point as their size (mass) increases. A larger molecule will have stronger Van der Waals forces and therefore require more energy to separate them. |
Revision
|
Practice test 1
|
|
| ||||
|
Practice test 2
|
| ||
EXTRA - Nanotechnology
Technology is constantly limited by the tools and materials that we have at our disposal. Recent discoveries and advancements in the field of nanotechnology have led to the challenge of finding new uses and applications for them.
"Nanotechnology is science, engineering, and technology conducted at the nanoscale, which is about 1 to 100 nanometers." (Nano.gov, 2015)
The properties of materials on the nano-scale can be very different to those found on the macro-scale. This opens up a whole new area of research called nanotechnology which could be extremely important in many areas of scientific development.
Innovation in the uses of nanomaterials has already lead to discoveries of materials such as graphene - one of the lightest and strongest materials that we know.
Innovation in the uses of nanomaterials has already lead to discoveries of materials such as graphene - one of the lightest and strongest materials that we know.
|
|
It also is an excellent conductor of heat and electricity and is very flexible. These kind of applications open up amazing possibilities in fields such as robotics and medicine.
The ability to design and build nanorobots has already started a revolution in the way that we view drug delivery systems by using things like nanotubes to deliver drugs to certain parts of the body. |
Although there are many advantages of the developments in nanotechnology, there are also many concerns such as:
- Health issues
- Environmental issues
- Regulation issues
Do you think the benefits outweigh the limitations?
References
Chemguide.co.uk,. (2015). atomic orbitals. Retrieved 28 September 2015, from http://www.chemguide.co.uk/atoms/properties/atomorbs.html
Chemwiki.ucdavis.edu,. (2015). Atomic orbitals. Retrieved 27 September 2015, from http://chemwiki.ucdavis.edu/@api/deki/files/8855/Single_electron_orbitals.jpg
Chemwiki.ucdavis.edu,. (2014). 6.9: Electron Configurations and the Periodic Table - Chemwiki. Retrieved 2 October 2015, from http://chemwiki.ucdavis.edu/?title=Textbook_Maps/General_Chemistry_Textbook_Maps/Map:_Brown,_LeMay,_%26_Bursten_%22Chemistry:_The_Central_Science%22/06._Electronic_Structure_of_Atoms/6.9:_Electron_Configurations_and_the_Periodic_Table
Jha, A. (2013). What is Heisenberg's Uncertainty Principle?. the Guardian. Retrieved 27 September 2015, from http://www.theguardian.com/science/2013/nov/10/what-is-heisenbergs-uncertainty-principle
Mpcfaculty.net,. (2015). Complete Electron Configurations. Retrieved 30 September 2015, from http://www.mpcfaculty.net/mark_bishop/complete_electron_configuration_help.htm
Nano.gov,. (2015). What is Nanotechnology? | Nano. Retrieved 5 November 2015, from http://www.nano.gov/nanotech-101/what/definition
Wwu.edu,. (2015). Stardust. Retrieved 27 September 2015, from http://www.wwu.edu/skywise/a101_dust.html
Chemguide.co.uk,. (2015). atomic orbitals. Retrieved 28 September 2015, from http://www.chemguide.co.uk/atoms/properties/atomorbs.html
Chemwiki.ucdavis.edu,. (2015). Atomic orbitals. Retrieved 27 September 2015, from http://chemwiki.ucdavis.edu/@api/deki/files/8855/Single_electron_orbitals.jpg
Chemwiki.ucdavis.edu,. (2014). 6.9: Electron Configurations and the Periodic Table - Chemwiki. Retrieved 2 October 2015, from http://chemwiki.ucdavis.edu/?title=Textbook_Maps/General_Chemistry_Textbook_Maps/Map:_Brown,_LeMay,_%26_Bursten_%22Chemistry:_The_Central_Science%22/06._Electronic_Structure_of_Atoms/6.9:_Electron_Configurations_and_the_Periodic_Table
Jha, A. (2013). What is Heisenberg's Uncertainty Principle?. the Guardian. Retrieved 27 September 2015, from http://www.theguardian.com/science/2013/nov/10/what-is-heisenbergs-uncertainty-principle
Mpcfaculty.net,. (2015). Complete Electron Configurations. Retrieved 30 September 2015, from http://www.mpcfaculty.net/mark_bishop/complete_electron_configuration_help.htm
Nano.gov,. (2015). What is Nanotechnology? | Nano. Retrieved 5 November 2015, from http://www.nano.gov/nanotech-101/what/definition
Wwu.edu,. (2015). Stardust. Retrieved 27 September 2015, from http://www.wwu.edu/skywise/a101_dust.html
| 1.4_if_tasks.pptx |